Integrated Pest Management and Insect Biology
Key to tables:
Table B–2: Comparison of green peach aphid, melon/cotton aphid, and foxglove aphid
Table B–4: Pesticide registration and toxicity
Table B–5: Mode of Action (MoA) classification
Table B–6: Insecticides, Miticides, and Molluscides
Integrated Pest Management
Consumer and grower concerns about widespread pesticide use, possible health risks from pesticide residues, problems with insecticide resistance, and groundwater contamination have led to increased interest in pest management programs that reduce use of broad spectrum, non-selective pesticides. A pest management program based upon Integrated Pest Management (IPM) strategies helps address these issues. Successful IPM programs combine accurate pest identification and scouting with cultural, biological and chemical controls in an economically and ecologically sound manner.
Pest Identification
Pest management decisions are initially based on correct/accurate identification and understanding of the arthropod (insect or mite) pest's life cycle (egg to adult). Effective pest management depends on a greenhouse grower's ability to determine which life stages are present and susceptible to pest management tactics. For example, spraying a pest control material (in this case an insecticide) to manage whiteflies is most effective when they are in the nymphal stages. Mis-identification of arthropod pests or their life stages can be costly and lead to inadequate control such that arthropod pest populations increase to levels that result in crop damage. Arthropod pest identification may be improved by participating in state-wide workshops and IPM training programs; by referring to manuals, picture guides and fact sheets; by using a text and image search engine such as Google; and by submitting specimens to an Extension entomologist or diagnostic laboratory (see Appendix II).
IPM Scouting and Decision-Making
Pre-Crop Site Evaluation
One month before introducing a crop, evaluate the entire greenhouse and surrounding area. Remove weeds, algae, "pet plants", and any plant or growing medium debris located throughout the greenhouse, particularly underneath benches, because these may provide refuge for certain arthropod pests. In addition, repair any drainage problems that may contribute to recurring arthropod pest outbreaks.
A fallow period (with greenhouses empty of crops and weeds) of at least four weeks may help to reduce pest pressure for the upcoming growing season. A break in production of as little as two weeks can help to reduce pest pressure.
Next, review previous pest problems in the greenhouse and current management strategies. Develop a plan of action to avoid or reduce these pest problems. You may easily prevent arthropod pest problems if you take the time to identify, analyze and correct problems before introducing crops.
Scouting
Scouting is the regular inspection of crops for insects, mites, diseases and cultural problems. The individual responsible for scouting could be an employee or an outside consultant. For employee scouts, it is best that scouting be the acknowledged responsibility, so that routine greenhouse tasks do not interfere with any scouting duties.
Scouting Tools
Helpful scouting tools include a 10x to 20x hand lens, Optivisor™, dissecting microscope, digital camera, sticky cards, flagging tape (of different colors), scouting forms, pH and electrical conductivity (EC) meters, disease detection kits (see www.agdia.com), and resource information (listed at the end of this section).
Inspection of Incoming Plants
Inspect incoming plant material for the presence of insects, mites, diseases, or cultural problems such as nutritional deficiencies. If feasible, quarantine infested or problematic plants in an isolated greenhouse or area so they can be treated with a pest control material (insecticide or miticide) before they are placed in production areas.
Scouting Program
Conduct regular weekly scouting through use of colored sticky cards, potato disks (to monitor for fungus-gnat larvae), random plant inspections, plant tapping and indicator plants.
Yellow and Blue Sticky Cards
Yellow sticky cards are commonly used in greenhouses to scout for or monitor insect pest populations. These cards capture adult whiteflies, thrips, fungus gnats, shore flies, leafminers, and winged aphids. Remember that mites, mealybugs, scales, and nonwinged aphids don't fly, so they are not captured on sticky cards. Also, it is important to note that many beneficial insects including parasitoids and predators may also be caught on yellow sticky cards. For information on identifying greenhouse insects, view the PowerPoint presentation, "Identifying Some Pest and Beneficial Species on Your Sticky Cards" on the UConn IPM Web site; go to www.ipm.uconn.edu and click on "greenhouse", then "general".
Position yellow sticky cards throughout the greenhouse, approximately 3-to-4 per 1,000-ft2 greenhouse space, or a minimum of one card per 1,000 ft2, with additional cards placed near openings such as doors, vents and sidewalls. Use clothespins and stakes to vertically attach sticky cards 4 to 6 inches (10 to 15 cm) above the crop canopy. As plants increase in height, move the sticky card upward (vertically) on the stake. Blue sticky cards are more attractive to thrips (and even shore flies) and can be used to detect low thrips populations on susceptible crops such as impatiens and begonias. However, thrips and other insect pests are easier to observe on yellow sticky cards than on blue sticky cards. When monitoring for fungus gnat adults, place yellow sticky cards horizontally near the growing medium surface because more fungus gnat adults will be captured compared to placing sticky cards vertically above the crop canopy.
Potato Disks
Use potato disks to monitor for fungus gnat larvae. Cut a fresh potato into disks 1.0 inch (2.5 cm) in diameter and 1/4 to 1/2 inch (0.6 to 1.2 cm) in thickness; then press the disks into the growing medium surface in tagged or flagged pots. For plug trays, you may cut potatoes into small "French fry" shapes or wedges and insert them into the growing medium. In general, use 5-to-10 potato wedges per 1,000 ft2 of greenhouse production area. After two days, inspect the undersides of the potato disks or wedges for the presence of fungus gnat larvae, which have distinct black head capsules. Record the number of larvae located on each potato disk or wedge, and those present on the surface of the growing medium.
Random Plant Inspections
In order to detect problems early, it is important to inspect crops at least weekly for feeding damage or presence of arthropod pest populations. When inspecting plants, select a set number or group of plants throughout the greenhouse (the actual number depends on scouting time and desired level of accuracy). Inspect leaves, stems, and roots. Inspect plants at floor, bench, and hanging basket levels. Sample a pre-determined number of each type of plant to increase the likelihood of locating "hot spots," which are areas with high arthropod pest populations. Take advantage of previous experience by focusing on plant species that tend to be susceptible to arthropod pests. Inspect plants for both pests and natural enemies (if applicable). If inspecting plants during the day, keep in mind that certain insect pests (such as black vine weevils or Asiatic garden beetles) and natural enemies such as rove beetles [(Atheta (=Dalotia)} or aphid midges (Aphidoletes) are more active at night.
Plant Tapping
Tap plant foliage or flowers over a sheet of white or black paper to monitor for arthropod pests such as aphid nymphs, thrips, spider mites, plant bugs and leafhopper nymphs.
Indicator Plants
Indicator plants are typically used to determine the effectiveness of pest management tactics or to monitor for the viruses (tospoviruses) such as impatiens necrotic spot virus (INSV) and tomato spotted wilt virus (TSWV), which are vectored by the western flower thrips (Frankliniella occidentalis). Before implementing any pest management strategy, select and tag (or flag) the leaves or stems of 1-to-5 infested plants per 1,000 ft2. Afterward, inspect the indicator plants to assess if arthropod pests have been killed. This makes it possible to evaluate the effectiveness and longevity of control or regulation.
To detect viruses transmitted by thrips, use either dwarf-fava-bean (Vicia faba) plants or 'Summer Madness' petunias. Position a blue card with the sticky portion covered near the indicator plants in order to attract adult thrips. If thrips adults possess any tospovirus, a brown, necrotic spotting will be observed near white feeding scars within 48 hours. Rogue out any infected petunia or fava-bean plants, to remove any potential viral sources. Virus infections are systemic in fava bean but not in petunia plants.
Tomato plants are a useful indicator plant to detect ethylene —a colorless, odorless gas— that can occur from the incomplete combustion of fossil fuel from greenhouse heaters that are not functioning properly, especially in tightly closed plastic greenhouses. Some growers place tomato transplants in each greenhouse when they begin heating in the winter. Tomato plants display downward bending of the leaves ("epinasty") when exposed to very low concentations of ethylene. Symptoms of ethylene damage vary depending upon plant species, but can include shedding of flower petals, flower bud abortion, leaf yellowing and necrosis.
Record Keeping
Each time the crop is scouted, record arthropod pest numbers, location within the greenhouse, and the number of plants inspected. Use a notebook or scouting form to record arthropod pest counts by date and to track population trends over time. Download a scouting form from the University of Connecticut's IPM Web site at www.ipm.uconn.edu; click on "greenhouse", then "general". Record data on arthropod pest abundance, location within the greenhouse, and population trends (increases, decreases, or stable) to help determine effectiveness of pest management tactics.
Making Pest Management Decisions
Each week, review scouting records to assess the effectiveness of your pest management tactics. Early detection of arthropod pests helps prevent the need to deal with extensive populations that may cause crop damage. It is also helpful to review scouting records at the end of each growing season to determine which arthropod pests were a problem and which pest management tactics provided the most effective control or regulation.
Pest Management in Greenhouses
Biological Control
Biological control is the use of living organisms (natural enemies) such as insects, mites, fungi or bacteria, to control or regulate pest populations. Greenhouses provide suitable temperatures, relative humidity and light for numerous biological control agents or natural enemies including parasitoids (parasitic wasps), predators, and entomopathogenic nematodes. Greenhouses in the northern regions of the U.S. are typically not subject to massive migrations of insect pests from outdoor crops. Greenhouse environment conditions including temperature, light, and relative humidity during the winter are often stable, which may influence the performance and persistence of natural enemies. Many natural enemies arecommercially available and can be incorporated into existing greenhouse pest management programs.
Some advantages of biological control agents include less exposure to toxic pest control material residues by the staff, no re-entry intervals (REI), and lower risk of spray damage or environmental pollution. Biological control is an important practice to prevent development of pesticide resistance.
Ornamental crop production in New England is complex because of multiple arthropod pests, potential biological control agents, and pest management strategies. Natural enemies cannot be used in the same manner as chemical insecticides or miticides. Pest control chemicals are typically applied after arthropod pests reach visible damaging levels. Effective pest control chemicals quickly reduce arthropod pest populations. Using natural enemies to cure or regulate pest populations is less successful than applying them preventively. Natural enemies should be released early in the cropping cycle when plants are small, arthropod pest populations are low, and before crop damage occurs. Multiple releases of natural enemies may be required throughout the growing season in order to manage arthropod pests at low population levels.
Some Guidelines for Success
A biological control program succeeds only if these steps are followed: 1) correctly identify all arthropod pests; 2) purchase natural enemies from a reliable biological control supplier; 3) make sure there is a consistent supply of high quality natural enemies; 4) emphasize that suppliers follow proper shipping procedures; 5) obtain directions from biological control suppliers on proper release rates and timing of applications; and compatability with pesticides 6) consult with suppliers of plant material to ensure that there are no long-lasting pest control chemical residues on incoming plant material (request a list of pest control materials applied from plant suppliers); 7) establish a regular and consistent scouting program; 8) develop a strategy based upon greenhouse production plans; 9) obtain full commitment of the owner and/or manager; and 10) ensure proper communication among all staff, employees and management regarding biological control programs.
Start any new biological control program in a small isolated greenhouse, in propagation houses, or in a greenhouse where edible crops such as herbs are grown. This approach allows you to gain experience before expanding into other production areas. It is critical to implement a scouting program and establish a favorable relationship with your biological control suppliers. The success of any biological control program relies on patience and a strong commitment to detail with an emphasis on scouting and record-keeping.
Arthropod pest identification is extremely important when initiating biological control programs in greenhouses because natural enemies, particularly parasitoids, are specific in the types of insect pests they use as hosts. For example, the aphid parasitoid Aphidius colemani attacks both the melon/cotton aphid (Aphis gossypii) and the green peach aphid (Myzus persicae), but does not attack the foxglove aphid (Aulacorthum solani). For arthropod pest identification information, consult trade journal articles, books, manuals, fact sheets, websites such as bugguide.net and picture identification guides, or send specimens to your Extension entomologist.
Planning Timeline
Plan carefully to ensure the success of your biological control program. Start planning 6-months to one year in advance if this is the first time you implement a biological control program. Contact suppliers, review your current pesticide usage, and move toward using pesticides that have a shorter residual for this transition period. Contact your plant suppliers before receiving plant materials, to determine what pesticides have been used on cuttings or liners. Develop a spreadsheet of dates when cuttings and plugs will arrive, your planting schedule, and when greenhouse plant production will begin to help pre-order natural enemies. You can order additional natural enemies if hot spots develop. Schedule delivery of the natural enemies, and consult with your supplier to decide whether a regular standing order or week-by-week order is needed.
Sources of Natural Enemies
Table B-1 provides contact information for seven biological control suppliers. Be sure to consult your biological control supplier(s) to determine availability of the natural enemy species you are interested in and the designated shipping requirements for them.
Product Quality
Natural enemies are living organisms that must be handled and stored carefully in order to maximize survival and sustain viability. Two important considerations associated with natural enemy quality are shipment arrival time and survivability upon arrival. In general, shipments of natural enemies should be received within four days after placing an order. Predatory mites such as Phytoseiulus persimilis that are shipped without a food source should be received after an overnight delivery. The package containing the natural enemies must be shipped in a sturdy container such as a polystyrene box that minimizes exposure to high and low temperatures. Request that the biological control supplier include ice packs and a data logger (if possible). Also, make sure the container is secured with good packing material during shipment. If appropriate, ask your biological control supplier(s) how to evaluate incoming shipments. Biological control suppliers often send a description of what to look for when receiving the natural enemies. For more specific information with color photos, see Grower Guide: Quality Assurance of Biocontrol Products compiled by Dr. R. Buitenhuis at: https://www.vinelandresearch.com/wp-content/uploads/2020/02/Grower-Guide.pdf
Upon receiving natural enemies, check the quality of the shipment. Assess the number of shipment days and how cold or warm the ice packs are. When you open the shipping container and retrieve the packages of natural enemies, consider the following: 1) Did you receive the correct natural enemy? That is, did you acquire what you ordered? 2) Did you receive the correct number of packages or units? Is each package or unit labeled with the estimated number of natural enemies? 3) Are the natural enemies alive? If a majority (>50%) of the natural enemies are dead, contact the biological control supplier immediately and request another shipment. Be sure to return the original shipment to the supplier.
If natural enemies shipped are mobile (for example, predaceous mites and some parasitoids), inspect them to determine if they are actively moving. To check natural enemies that are shipped as eggs or pupae, place a small sample in a shaded, unsprayed area for 2-3 days and then look for the active larvae or adults.
Use the following procedures to assess the quality of predatory mites, whitefly parasitoids, and entomopathogenic nematodes.
Predatory Mites
After receiving a shipment of predatory mites such as Phytoseiulus persimilis or Neoseiulus (Amblyseius) cucumeris, evaluate a small sample of the contents to see whether the mites are alive, and whether you received the correct quantity. For example, if by volume, the entire package fills five Styrofoam coffee cups, select 1/8 cup or 2.5% of the material, after first gently rotating the contents of the package to mix the predatory mites with the bran carrier. Pour the 1/8-cup of material onto a sheet of white paper (8 x 11 inch, or 20 x 28 cm) and gently spread the contents out using a soft camel-hair paint brush. Count the number of mites that move to determine the viability of the package. If no active mites are observed, immediately contact your biological control supplier to discuss appropriate procedures.
Shipments of N. cucumeris may also contain grain mites (Acarus siro), which serve as a food source. Grain mites are white, have long protruding hairs on their backs and are generally less mobile than the tan-colored fast moving N. cucumeris. Avoid counting any grain mites during the quality evaluation process. You should also check the rim of the container in which the predatory mites are shipped for the presence of live individuals.
Whitefly Parasitoids
Shipments of whitefly parasitoids contain either paper cards with a predetermined number of pupae per card (e.g., 30 to 100 pupae per card for Encarsia formosa) or an estimated number of pupae in loose bran or sawdust (e.g., Eretmocerus eremicus) or blister packs (small tags with a plastic chamber containing the loose pupae with an opening on the reverse side so adult wasps can emerge). To assess whitefly parasitoids shipped as pupae on paper cards, place a single card into a glass jar with a tight-sealing lid. Expose the jar to room temperature (70 to 75°F, or 21 to 24°C) and position out of direct sunlight. Do not disturb the jar for approximately two weeks. Add a piece of yellow sticky card in the container for easier counting. After this time, count or estimate the number of adult parasitoids that have emerged from the pupae. Make sure ≥95% of adult parasitoids have emerged. For E. eremicus, which is sold as loose pupae, place a subsample (approximately 2% by volume of the total contents) or blister pack into a jar with a tight-sealing lid. After storing at the same temperatures and time as described above for E. formosa, count the number of live adult parasitoids that emerge from the pupae. Again, make sure ≥95% of adult parasitoids emerge. If <90% of the adult parasitoids emerge from the pupae, contact your biological control supplier immediately.
Entomopathogenic nematodes
After receiving a shipment of entomopathogenic or beneficial nematodes (e.g., Steinernema feltiae), it is important to assess quality before application. To do this, place a small quantity of the product (5 ml) into a shallow container or Petri dish. Add one or two drops of tepid water, wait 30 minutes, and then look for actively moving nematodes, which have a slight "J" or "S" curvature or may be curled up into a "donut" shape. If the nematodes appear "straight" like a toothpick, they are not viable. It is easier to observe the small nematodes (0.02 inch, or 0.6 mm in length) when the container is placed on a black background or shine a flashlight on them. Consult your biological control supplier if you have any questions.
Storage
Most natural enemies have a very short shelf life. Those that are shipped without a food source should be applied immediately. Natural enemies that are shipped with a food source can be stored for a few days before use; however, it is highly recommended to release natural enemies immediately upon arrival. Storage time depends on the particular natural enemy and environmental conditions. If natural enemies must be stored, maintain them at 50 to 60°F (10 to 15°C) and 50 to 70% relative humidity. Do not put parasitoids or predators in refrigerators or in direct sunlight since refrigerator temperatures (35 to 45°F or 1 to 7°C) and exposure to sunlight are detrimental to both natural enemy types. In general, the longer that natural enemies are stored, the lower the survivability of the individuals. One common cause of natural enemy mortality during storage is desiccation (drying), which may be avoided by placing containers of natural enemies in a plastic bag along with a moist sponge, and then securely sealing the bag. Entomopathogenic nematodes in unopened containers may be stored for up to 10 weeks at temperatures of 40°F (4°C). Although refrigeration is not critical, it is still recommended; however, be sure to avoid exposure to freezing temperatures, which can kill the nematodes. Avoid storing the nematodes in a refrigerator that is opened frequently, as constant temperatures are best. When planning on using the entomopathogenic nematodes, place them in room temperature for 1 to 2 hours before placing into the water. Too drastic a temperature change from the refrigerator to the water will stress or even kill them.
Release of Natural Enemies
The purchase and release of natural enemies into greenhouses is referred to as augmentative biological control. There are two augmentative biological control strategies: inoculation and inundation.
Inoculation
Inoculation is the release of small numbers of natural enemies early in the growing season or cropping cycle with the intention that the natural enemies will establish and reproduce in the greenhouse, providing long-term control or regulation. An example of inoculation is releasing the parasitoid Encarsia formosa to control greenhouse whitefly, Trialeurodes vaporariorum, on greenhouse-grown tomatoes.
Inundation
Inundation is the release of high numbers of natural enemies into a greenhouse to provide short-term control or regulation. Additional releases may be required during the growing season or cropping cycle in order to lower pest populations. Examples of this release strategy include drenching growing media with entomopathogenic nematodes (Steinernema feltiae) to control fungus gnat larvae, or weekly releases of the parasitoid Eretmocerus eremicus to suppress populations of the sweet potato whitefly, Bemisia tabaci.
Release Rates
The two most important factors for success of a greenhouse biological control program are release rate and application frequency. Consult biological control suppliers or your Extension entomologist for information about when and how to apply natural enemies in order to control or regulate arthropod pests of ornamental plants. Consult your supplier for recommended release rates, which are typically based on area (e.g., amount per ft2 or m2), and vary depending upon the severity of the arthropod pest infestation, type of natural enemy released (e.g., parasitoid or predator), crop type, and plant height.
Detailed information regarding the release of natural enemies for specific arthropod pests in New England greenhouses is presented later in this section.
Banker Plants
Banker plants provide an innovative way to introduce and support biological control agents. They are used to rear prey that, although specific to the banker plant, provides a continuing food source for specific natural enemies to ensure that they continually reproduce. Most banker plants are used for aphid management, although some banker plant systems have been developed to manage other arthropod pests such as thrips, mites, and whiteflies. Do not apply fungicides, plant growth regulators or insecticide drenches to the banker plants. Be sure to remove banker plants from the greenhouse before applying any spot sprays in the greenhouse.
Aphid Banker Plants
Banker plants for aphids are containers of wheat (Triticum aestivum) or winter barley (Hordeum vulgare), on which colonies of grass-feeding aphid species such as the corn-leaf aphid (Rhopalosiphum maidis), greenbug (Schizaphis graminum), and/or the bird-cherry aphid (Rhopalosiphum padi) are established. It is helpful to select plant varieties that are resistant to powdery mildew because greenhouse conditions in the spring are often favorable to powdery mildew fungi.
Banker plants may be placed along walkways and at the ends of benches. It is essential to evenly distribute them throughout a greenhouse. Some greenhouse growers place banker plants at hanging basket level with drip irrigation to ensure that the banker plants are well irrigated and fertilized without inadvertently washing the aphid natural enemies off the banker plants, as may happen with overhead irrigation. Generally, banker plants should be placed approximately 131 ft (40 m) apart, using 4 to 5 banker plants per 10,000 ft2, in order to increase parasitization. Furthermore, it has been recommended to distribute containers of wheat or barley with the grass-feeding aphid, among the main crop at a rate of one banker plant per 1,000 ft2 even before aphids are detected. It should be noted that existing recommended rates may vary since limited research has been conducted. Start with these rates and adjust in succeeding years based on your experience. Banker plants must be placed close together within a given area in order to allow parasitoids such as Aphidius colemani to find prey on plants, because research has found that these parasitoids migrate only 3.2 to 6.5 ft (0.9 to 2.0 m) from the point of their release.
"Starter aphid colonies" can be ordered from several biological control suppliers (See Table A-1). Place orders for banker plants up to 6 weeks before you start plants in the spring and when aphids are expected in the greenhouse based on previous experience. Transplant the plugs or "starter aphid colony" into large pots (10 inch or 25.4 cm or larger) so that the banker plants have plenty of room to grow. Wait one or two weeks for the non-pest aphid populations to increase. It is necessary to protect the starter banker plants from natural enemies (either established in the greenhouse or from naturally occurring natural enemies that may enter the greenhouse from outdoors) to successfully build up the population of non-pest cereal aphids before releasing A. colemani. Aphid banker cages can be ordered online or growers may build their own. The cages should be double sealed and covered with small mesh such as thrips screening to ensure the necessary ventilation without having the parasitic wasps enter the banker cage.
After positioning the banker plants in the greenhouse, order a shipment of A. colemani if you have green peach or melon aphid, and release the "aphid mummies" or adults immediately onto the banker plants. Aphidius colemani parasitizes the grass-feeding aphid, which is not a pest of most greenhouse-grown crops, with the exception of certain ornamental grasses and other monocots such as orchids, iris, spring bulbs, onions and garlic. Some growers have noticed that bird cherry aphids have moved onto Cordyline, Cyperus and Dracaena as well. Place a fresh banker plant next to an inoculated banker plant every 2 to 3 weeks.
It should be noted that the banker plant system may not be appropriate for use with all parasitoids. For example, the bird-cherry aphid is too small to support development of the parasitoid, A. ervi. Check banker plants weekly and look for newly parasitized aphids ("aphid mummies"), which indicate that the parasitoids are establishing on the banker plants.
Banker plants can sustain non-pest cereal aphid populations at low levels. However, A. colemani is not effective against the foxglove aphid. If this aphid is the predominant pest aphid, use the predatory midge, Aphidoletes aphidimyza. It is important to regularly start new banker plants routinely since they decline and die within a few weeks. Be sure to prevent the grasses from drying out. Inoculate new banker plants by physically transferring aphids from old banker plants onto new ones every 2 to 3 weeks. In general, banker plants are most effective during spring production. Destroy aphid banker plants at the end of the season to avoid the buildup of hyperparasites (a parasite whose host is another parasite, in this case, A. colemani).
Thrips Banker Plants
Orius spp., the minute pirate bug, is a generalist predator that feeds on immature and adult thrips, spider mites and pollen. Because minute pirate bugs are slow to establish in greenhouses, different types of banker plant systems have been investigated.
Minute pirate bugs are released onto ornamental pepper plants that are in flower (with pollen as a food source) which serve as banker plants. Some suppliers recommend 80 to 100 pots per acre with 3 plugs of ornamental peppers per pot. Research in Canada reported that the ornamental pepper cultivar 'Purple Flash' was a more effective banker plant than the cultivar 'Black Pearl' because they produce more pollen.
Advance planning is needed when using pepper banker plants. Orius can be introduced as early as March or April under natural daylength conditions. Release about 60 to 80 minute pirate bugs per flowering pepper plant. Release weekly for 4 to 6 weeks. It may take up to 2 months to establish sufficient populations. Therefore, Neoseiulus cucumeris is released unto the pepper plants to prevent outbreak.
Minute pirate bug females lay eggs in or near the pepper flowers. As long as the ornamental pepper plants are in flower and producing pollen, minute pirate bugs will reproduce on the banker plants. However, to increase their egg laying, one can release Ephestia (sterile moth) eggs as a supplemental food source. One disadvantage of this system is that the pepper fruits must be removed in order to keep the plants continually flowering, and this could be very labor intensive. Some growers use Lobularia Stream series with their ornamental pepper plants. Lobularia is attractive to minute pirate bugs and hover flies or syrphid flies.
A less widely used banker plant system is the use of mullein (Verbascum thapsus) banker plants to rear Dicyphus hesperus, a generalist predator that feeds upon greenhouse whiteflies and mites. Mullein plants need to be grown for 10 to 12 weeks before incorporating them into the main crop. D. hesperus populations are slow to establish and this can take up to 2 months. D. hesperus also feeds upon plant fluids, however, populations can be increased by using Ephestia (sterile moth) eggs as a supplemental food source.
Habitat Plants
In outdoor production areas, you can conserve natural enemies by reducing the use of broad-spectrum pesticides and by using spot treatments based upon scouting results. Select multiple types of flowering plants (as sources of pollen and nectar) for adult natural enemies, to help to attract and conserve natural enemies that are naturally occurring and also those that have been released.
Some New England greenhouse growers have been trialing different types of "habitat planters" to help sustain minute pirate bugs. They have observed that Cosmos 'Sensation White' has been especially effective. Their habitat planters have also included achillea, lantana, alyssum, catmint and dill. Minute pirate bugs are released on the planters along with predatory mites including Neoseiulus (= Amblyseius) cucumeris. Other naturally occurring natural enemies may also be attracted to these habitat planters.
For more information on identifying beneficial insects, see references listed at the end of this chapter. However, it should be noted that habitat planters, in addition to attracting natural enemies, may also attract a variety of insect pests.
Table B-1: Commercially available natural enemies for the major arthropod (insect and mite) pests of greenhouse-grown crops.
For additional suppliers of natural enemies, see: Association of Natural Biocontrol Producers: www.anbp.org
| Suppliers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pest | Biological Control Agent | Applied Bionomics | Beneficial Insectary | BioBee | Biobest | Bioline Agrosiences | IPM Labs | Koppert |
| Aphids | Adalia bipunctata | x | ||||||
| Aphids | Aphelinus abdominalis | x | x | x | x | x | ||
| Aphids | Aphidius colemani | x | x | x | x | x | x | |
| Aphids | Aphidius ervi | x | x | x | x | x | x | |
| Aphids | Aphidius matricariae | x | x | x | x | |||
| Aphids | Aphidoletes aphidimyza | x | x | x | x | x | x | x |
| Aphids | Aphid Banker Plant | x | x | |||||
| Aphids | Chrysoperla spp. | x | x | x | x | x | x | |
| Aphids | Hippodamia convergens | x | ||||||
| Armored Scales | Rhizobius (Lindorus) lophanthae | x | ||||||
| Broad mites | Neoseilus (Amblyseius) californicus | x | x | x | x | x | ||
| Broad mites | Neoseilus (Amblyseius) cucumeris | x | x | x | x | x | x | x |
| Broad mites | Amblyseius swirskii | x | x | x | x | x | x | |
| Caterpillars | Trichogramma brassicae | x | x | x | x | |||
| Caterpillars | Trichogramma minutum | x | x | |||||
| Caterpillars | Trichogramma pretiosum | x | ||||||
| Citrus mealybugs | Anagyrus pseudococci | x | x | |||||
| Citrus mealybugs | Cryptolaemus montrouzieri | x | x | x | x | x | x | |
| Cyclamen mites | Neoseilus (Amblyseius) californicus | x | x | x | x | x | ||
| Cyclamen mites | Neoseilus (Amblyseius) swirskii | x | x | x | x | x | x | |
| Cyclamen mites | Neoseilus (Amblyseius) cucumeris | x | x | x | x | x | x | x |
| Fungus gnats | Dalotia coriaria | x | x | x | x | x | x | |
| Fungus gnats | Stratiolaelaps scimitus | x | x | x | x | x | x | x |
| Fungus gnats | Steinernema feltiae | x | x | x | x | x | x | |
| Leafminers | Dacnusa sibirica | x | x | |||||
| Leafminers | Diglyphus isaea | x | x | x | x | x | x | |
| Leafminers | Steinernema feltiae | x | x | x | x | x | x | |
| Shore flies | Dalotia coriaria | x | x | x | x | x | x | |
| Shore flies | Steinernema carpocapsae | x | x | x | x | x | x | |
| Spider Mites | Amblyseius andersoni | x | x | x | x | |||
| Spider Mites | Feltiella acarisuga | x | x | x | x | x | ||
| Spider Mites | Galendromus occidentalis | x | ||||||
| Spider Mites | Mesoseilus longipes | x | ||||||
| Spider Mites | Neoseilus (Amblyseius) californicus | x | x | x | x | x | ||
| Spider Mites | Neoseiulus fallacis | x | x | x | ||||
| Spider Mites | Phytoseiulus persimilis | x | x | x | x | x | x | x |
| Spider Mites | Stethorus punctillum | x | x | |||||
| Western flower thrips | Amblyseius swirskii | x | x | x | x | x | x | |
| Western flower thrips | Amblyseius limonicus | x | x | |||||
| Western flower thrips | Dalotia coriaria | x | x | x | x | x | x | |
| Western flower thrips | Stratiolaelaps scimitus | x | x | x | x | x | x | x |
| Western flower thrips | Neoseilus (Amblyseius) californicus | x | x | x | x | x | ||
| Western flower thrips | Neoseilus (Amblyseius) cucumeris | x | x | x | x | x | x | x |
| Western flower thrips | Orius spp. | x | x | x | x | x | ||
| Western flower thrips | Steinernema feltiae | x | x | x | x | x | x | |
| Whiteflies | Amblyseius swirskii | x | x | x | x | x | x | |
| Whiteflies | Chrysoperla spp. | x | x | x | x | x | x | |
| Whiteflies | Delphastus pusillus (catalinae) | x | x | x | x | x | ||
| Whiteflies | Dicyphus hesperus | x | ||||||
| Whiteflies | Encarsia formosa | x | x | x | x | x | x | x |
| Whiteflies | Eretmocerus eremicus | x | x | x | x | x | x | |
Cultural Control
Biological control can only succeed when integrated with proper cultural control and sanitation practices. In fact, stringent implementation of these two strategies significantly minimizes potential problems associated with arthropod pests, and enhances the effectiveness of natural enemies.
Follow these cultural control and sanitation practices to reduce arthropod pest problems:
-
Avoid over-fertilizing plants, particularly with nitrogen, since this encourages the development and reproduction of arthropod pests such as aphids, whiteflies, mealybugs and spider mites.
-
Avoid under- and over-watering plants, as this places undue stress on plants, thereby increasing susceptibility to arthropod pests.
-
If possible, grow less susceptible plants or cultivars.
-
Remove weeds and old growing medium debris from both inside and around the perimeter of the greenhouse.
-
Discard heavily infested plants into disposal or garbage containers with tight-sealing lids in order to prevent adult whiteflies, fungus gnats, thrips and winged aphids from migrating back onto the crop. Or, place all debris into a plastic bag and immediately remove from the greenhouse.
-
Sanitize tools, equipment, work areas, benches and pots with commercially available disinfectants to prevent contamination from arthropod pests and diseases.
Exclusion
Exclude insect pests by installing screening material (fine mesh screens or microscreening) over greenhouse openings such as intake vents, side walls, and ridge and louver vents. This prevents or inhibits insects such as adult thrips, whiteflies, leafminers and winged aphids from migrating into greenhouses, thus allowing natural enemies to deal with already existing insect pest populations. It is critical to properly size the screening material since the small openings (or mesh) may disrupt airflow, making it difficult to sufficiently cool the greenhouse. For more information, contact the National Greenhouse Manufacturer's Association (www.ngma.com).
Pest Control Materials
If natural enemies alone fail to provide sufficient control or regulation of a given arthropod pest population, or when more than one arthropod pest population is prevalent on a crop simultaneously, then the use of pest control materials (insecticides or miticides) may be warranted. When selecting any material, try to use one that has a low toxicity to workers such as the alternative (or selective) pest control materials. Alternative pest control materials include insect growth regulators, insecticidal soaps (potassium salts of fatty acids), horticultural oils (petroleum or neem-based), selective-feeding blockers, microbial agents, and related microorganisms such as spinosad (Saccharopolyspora spinosa). For some arthropod pests there are no effective natural enemies, or a greenhouse grower may prefer to use a pest control material. See Table B-5 for pest control material recommendations.
Pest Control Materials and Natural Enemies
The use of natural enemies in conjunction with pest control materials depends on a number of factors such as whether the natural enemy is a predator or parasitoid (parasitoids including Encarsia formosa may be more sensitive to wet sprays or residues than predators), the particular species and life stage of the natural enemy, the rate used, timing of application, and mode of action of the specific pest control material. Furthermore, the characteristics of alternative pest control materials and method of application must be considered when attempting to integrate natural enemies with the use of these pest control materials. Pest control materials may either kill natural enemies directly or affect them indirectly. If pest control materials kill their food source or prey then natural enemies may starve or disperse from the area. Parasitoids may also be killed while developing inside their hosts. Certain pest control materials may have repellent activity, which may influence the ability of natural enemies to locate prey.
Pest control materials may also indirectly affect the ability of natural enemies to survive and reproduce. In fact, some pest control materials may actually sterilize females, reduce their ability to lay eggs, or modify the sex ratio so there are fewer females. However, the indirect impact of pest control materials may vary depending on whether the pest control material is an insecticide, miticide or fungicide; the mode of action of the pest control material; the application rate; and the application method. More information is generally available about the impact of insecticides and miticides than about either fungicides or plant growth regulators on natural enemies. It is important to note that any harmful effects may not be caused by the active ingredient of the pest control material, instead by inert ingredients such as additives, surfactants, carriers and/or solvents. For example, xylene, which is present in the formulation of the insect growth regulator kinoprene (Enstar AQ), may be toxic to some natural enemies, especially parasitoids.
In general, alternative pest control materials may be more selective and less harmful to natural enemies than conventional pest control materials. However, wet sprays of insecticidal soaps and horticultural oils are generally toxic to many natural enemies, especially parasitoids (although spray residues are less harmful after they have dried). In addition, fungicides with broad modes of activity may be more detrimental to natural enemies than fungicides with site-specific modes of action.
It is important to avoid using pest control materials in the organophosphate, carbamate, and pyrethroid chemical classes (often referred to as conventional pest control materials) prior to releasing natural enemies, since the residues of many materials in these chemical classes can persist for up to four months in the greenhouse. Often, a transition period may be needed, when shorter residual pest control materials are used, before starting a biological control program.
Consult Side Effect Databases
For more information on the compatibility of pest control materials with natural enemies, refer to online resources, such as:
(1) Koppert's online interactive database: https://www.koppert.com/side-effects/
(2) Biobest: http://www.biobestgroup.com/ (click on "side effect manual")
(3) Bioline Agrosciences: https://www.biolineagrosciences.com/ and download Bioline App
(4) BASF (formerly Becker Underwood): http://betterplants.basf.us/ (click on "indoor greenhouses", then under product type "beneficial nematodes" click on "Nemasys beneficial nematodes", then click on "Nemasys chemical compatibility guide").
(5) BioWorks: for Botanigard biocompatibility information, go to https://www.bioworksinc.com/wp-content/uploads/products/shared/BiologicalControlAgents.pdf
Research is continuing on the compatibility of pest control materials with natural enemies so be sure to contact your university-based ornamental Extension or research entomologist or biological control supplier.
Insect And Mite Pests
A multitude of arthropod pests (insects and mites) may be encountered on greenhouse-grown crops. The type of arthropod pest or pests that may be a problem throughout the growing season depends on several factors including geographic location, plant material grown, environmental conditions (e.g., temperature, light and relative humidity), and cultural practices. This section discusses the following arthropod pests of greenhouse crops: aphids, beetles, caterpillars, fungus gnats, leafhoppers, leafminers, mealybugs, mites, plant bugs, sawflies, scales, shore flies, thrips, weevils and whiteflies. Potential related pests are also discussed including millipedes, springtails, slugs and snails, sow bugs and symphylans.
Aphids
Identification, Biology and Life Cycle
More than 30 aphid species feed on greenhouse-grown crops. The species encountered depend on the crops grown and geographic location. Aphids, in general, are small (1/8 inch or 3.0 mm long), soft-bodied insects with long legs and antennae, and two tube-like protrusions on the abdomen called cornicles (See Figure B-1 below). Aphids vary in color depending on the plants they feed on, so do not rely on color to identify aphids. Aphids typically occur in large colonies on new terminal growth, base of buds, or undersides of mature leaves. Some aphids may occasionally be found on plant roots. Aphids use their piercing-sucking mouthparts to remove plant fluids. They excrete excess fluid as honeydew, which is a clear, sticky liquid that attracts ants and serves as a growing medium for black sooty mold fungi. Typical symptoms of aphid feeding include plant stunting, leaf yellowing, and distortion of plant growth. As they molt, they leave behind white cast skins. In addition, several aphid species transmit destructive viruses to plants.
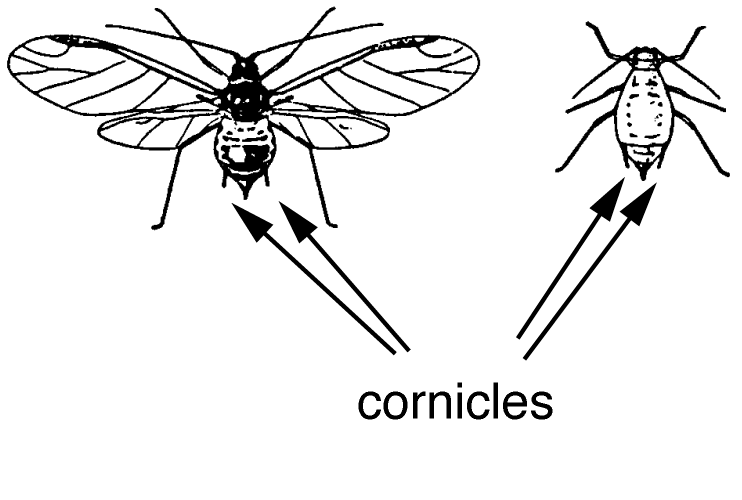
Figure B-1: Winged and wingless forms of green peach aphid
Aphid species in outdoor environments reproduce sexually by laying eggs on plants. Aphids in the greenhouse reproduce asexually, with unmated adult females giving birth to live offspring that are all female (this is referred to as parthenogenesis). This biological characteristic, coupled with the high reproductive ability of female aphids (some species give birth to as many as 100 nymphs over a 20- to 30-day period), explains why aphid populations can increase so rapidly. As the aphid colony increases in age and size, more winged adults occur.
If high aphid populations are present on plants or if plant nutritional quality declines, then winged adult females will develop within the population. This allows aphids to disperse to plants that are less crowded and provide adequate food. In outdoor environments, many aphid species overwinter as eggs. However, in greenhouses, aphids continue to develop and reproduce throughout the year.
The common aphid species that are encountered in greenhouses include the green peach aphid (Myzus persicae), melon/cotton aphid (Aphis gossypii), foxglove aphid (Aulacorthum solani, also known as glasshouse potato aphid), and root aphid (Pemphigus spp.). Occasionally, potato aphids (Macrosiphum euphorbiae) may be found, especially on solanaceous crops.
Table B-2 Comparison of green peach aphid, melon/cotton aphid, and foxglove aphid.
|
Green Peach Aphid |
Melon/ Cotton Aphid |
Foxglove Aphid |
|
|
Color |
light green; occasionally pink to orange |
variable from dark green to light green to yellow |
pale green |
|
Cornicles (tailpipes) |
light green; slightly darker than the body, with black tips |
black |
green spot at base |
|
Head (use hand lens to see profile of top of head) |
indentation |
no indentation |
indentation |
Table reproduced with permission from from Integrated Pest Management for Bedding Plants: A Scouting and Pest Management Guide, copyright 1999, New York State Integrated Pest Management Program, Cornell University.
Following are descriptions of the common aphid species encountered in greenhouses:
A. Green peach aphid: This aphid species can be distinguished from the melon/cotton aphid by the length and color of the cornicles (the tube-like protrusions extending from the end of the abdomen). Only the tips of green peach aphids' cornicles are black. Also, the head has a distinct indentation at the base of the antennae, which are approximately as long as the body.
B. Melon/cotton aphid: The cornicles of melon/ cotton aphid are short and black. Wingless adults vary in color from light yellow to very dark green (making them appear black). Melon/cotton aphids do not have a distinct indentation at the base of the antennae like the green peach aphid.
C. Foxglove aphid: Foxglove aphids have green flecks at the base of their cornicles, and black markings on their leg joints and antennae. Foxglove aphids are pale green to yellow, with a somewhat "shiny" appearance compared to other aphids. They also tend to fall off plants when disturbed and they can cause severe leaf distortion, more so than the green peach and melon/cotton aphid.
D. Potato aphid: Potato aphids are large and slender, with long cornicles, legs and a long cauda (structure at the rear of the body). They are often green but may be pink or red depending upon food source. Wingless nymphs have a longitudinal stripe down their back. Antennae are longer than their body and the eyes may be bright red.
E. Root aphid: The primary root aphid (Pemphigus species) overwinters as eggs and infests plants such as sedum, veronica, aster, coreopsis, and sempevirens in spring and fall. Root aphids may be misidentified as mealybugs because they are covered with white wax, but they are smaller than mealybugs. Root aphids have reduced ring-like cornicles, which are located on the end of the abdomen. These cornicles may be seen under magnification.
For more information, see "Common Greenhouse Pests: Aphids" at https://www.uvm.edu/~entlab/Greenhouse%20IPM/Pests&Beneficials/Aphids.pdf
Scouting
Aphids feed on a wide range of host plants including herbaceous annuals and perennials, herbs and vegetable bedding plants. Concentrate efforts on species/cultivars that are most susceptible to aphids.
Signs of an aphid infestation include the presence of white cast (molted) skins, honeydew, and black sooty mold fungi. Ants may be attracted to the honeydew. Yellow sticky cards capture winged aphids that have entered the greenhouse from outdoors, particularly during spring and early summer. Since most aphids are wingless, the use of yellow sticky cards is not a reliable indicator of aphid populations in the greenhouse. Direct visual crop inspection is required. Be sure to inspect undersides of the lower leaves, leaf stems and flowers for signs of aphids.
Cultural Control
Inspect incoming plants for aphids. Avoid high nitrogen fertilization that promotes lush growth that is favorable to aphids. Remove weeds and "pet plants" that can be sources of aphid infestations. Screen doors and vents in spring and fall.
Biological Control
Aphids are susceptible to many natural enemies, including predators and parasitoids, and entomopa-thogenic fungi. In general, predators do not effectively maintain aphid populations at acceptably low levels, with the possible exception of the predatory midge, Aphidoletes aphidimyza, which is not effective under short-day conditions.
Aphid Predators
A. Ladybird beetle: The convergent ladybird beetle, Hippodamia convergens, and the two-spotted lady beetle Adalia bipunctata are commercially available from some biological control suppliers. Both larval and adult stages of ladybird beetles feed on nymphs and adults of aphids. However, they may not distinguish between parasitized and nonparasitized aphids. Adult beetles feed on pollen, fungi, and nectar in the absence of prey. Ladybird beetles are generalist predators and, in addition to aphids, may feed on thrips, whiteflies, mealybugs and scales. Because ladybird beetle adults can fly, they are difficult to establish in greenhouses. Release ladybird beetle adults near aphid colonies, in the evening or early morning when the vents are closed. Repeat applications may be needed. When scouting the crop, look for aphids that have been fed upon and ladybird beetle adults, larvae, and/or eggs. Eggs are typically bright yellow and are laid in clusters near aphid colonies.
B. Green lacewing: The green lacewing, Chrysoperla spp. (C. rufilabris and C. carnea), are adapted to the environmental conditions (e.g., temperature, relative humidity and light intensity) typically present in greenhouses. Adults are usually active at night and feed on nectar, pollen, and honeydew. Green lacewing larvae (referred to as "aphid lions") feed on many aphid species as well as mites, whiteflies and mealybugs. The larvae, which are cannibalistic, must be distributed over a wide area throughout the production facility. Larvae may survive better than eggs, and are quicker acting. They are available mixed with an inert ingredient such as rice hulls. They should reduce the aphid population after about two weeks. Repeat applications are typically required, and control or regulation may be hampered if low aphid populations are present. Lacewings may also be less effective on plants with hairy leaves. Scouting for green lacewings is difficult because the larvae tend to hide within the plant canopy during the day. Adults are active at night. Examine leaf undersides for the presence of eggs, which are laid on extended stalks.
C. Predatory midge: The predatory midge, Aphidoletes aphidimyza, feeds on over 60 aphid species. The midge is nocturnal (active at night), and prefers to reside in dark, humid areas near the lower plant canopy. Only the larval stage is predaceous. Adults feed primarily on pollen and honeydew. The bright orange larvae kill aphids by biting their knee joints, injecting a paralyzing toxin and then withdrawing the internal body fluids. Aphidoletes aphidimyza is typically sold as pupae in bottles or blister packs. Adults that emerge from the pupae lay eggs near aphid colonies. Larvae descend to the ground (below greenhouse benches) to pupate, so this predator may be less effective in greenhouse facilities with concrete floors. Aphidoletes aphidimyza is most effective during the summer because exposure to short days and low temperatures during winter induces diapause (a period of inactivity or rest). Initiate releases in the evening near aphid colonies when fans are off and vents are shut. Greenhouse temperatures should be 60 to 80°F (15 to 26°C), with 50 to 85% relative humidity. Aphidoletes aphidimyza may be used in combination with aphid parasitoids. When scouting, look for fed-upon aphids, which may appear shriveled and brown or black. The adults are rarely seen, since they are active at night.
D. Naturally occurring predators: Hover flies such as Episyrphus balteatus, also known as syrphid or flower flies, are naturally occurring beneficial, predatory insects that may enter the greenhouse from outdoors. Adults begin emerging in April and May, about the same time that aphid populations start to increase outdoors. Hover flies are named for the adults' ability to hover in mid-air, dart a short distance very quickly, and then hover again.
Adults are 3/8 to 3/4-inch long (9.5 to 19 mm) and resemble small bees or wasps, but they are members of the fly (Diptera) family, with only two wings. Hover flies have short antennae and large eyes. The pollen-feeding adults may be seen hovering around flowers or aphid colonies (including aphid banker plants) where they lay eggs that resemble small grains of rice. Eggs hatch into small, legless larvae that may be mistaken for caterpillars, and that feed on aphids and thrips. Larvae vary in color, are legless and have a tapered head. They may leave tar-like excrement on plant leaves. Many growers use lobularia with their ornamental pepper banker plants to attract hover flies.
Aphid Parasitoids
In general, parasitoids reduce aphid populations more effectively than predators, although parasitoids may fail to provide acceptable control or regulation under warm conditions when aphid populations tend to increase rapidly. It may be difficult for aphid parasitoids to search effectively on some ornamental crops with sticky leaves. Four parasitoids are commercially available (see Table B-3 on page B.14). Aphid parasitoids are host-specific in terms of the aphid species they attack. Mixtures of parasitoid species are commercially available and should be used when multiple aphid species are present. Parasitoids are shipped as "aphid mummies" from which parasitoid adults soon emerge. To increase parasitoids' effectiveness, place small groups of the aphid mummies in cups near aphid colonies. Release them at the end of the day in shaded locations. Do not let aphid mummies get wet. Release rates may vary depending on the parasitoid species.
A. Aphidius colemani is a tiny (2 mm long) wasp that is used against green peach aphids and melon aphids. The adult wasp lays one egg inside an aphid. This egg hatches into a larva that feeds inside the aphid. When mature, a new adult wasp emerges from the tan aphid mummy. This active searcher is not as effective at temperatures above 86°F (30°C). (See "Aphid Banker Plants" on page B.6 for more information).
B. Aphidius ervi attacks larger aphids such as the foxglove aphid (Aulacorthum solani) and potato aphid (Macrosiphum euphorbiae). It resembles A. colemani but is about twice as large and darker in color.
C. Aphidius matricariae attacks green peach aphids (Myzus persicae) and the closely related tobacco aphid.
D. Aphelinus abdominalis attacks foxglove aphid (Aulacorthum solani) and potato aphid (Macrosiphum euphorbiae). Adults feed on the small aphid nymphs and parasitize the larger aphids. Aphelinus is better able to withstand higher temperatures than Aphidius species. Look for elongated black mummies that are less swollen than Aphidius mummies.
Greenhouse temperatures should be 65 to 77°F (18 to 25°C), with 70 to 85% relative humidity. Aphid parasitoids must be applied preventively to suppress or regulate aphid populations. They are less effective when aphid populations are high and already causing plant damage. Release parasitoids on a regular basis to sustain their populations during the growing season. Remove yellow sticky cards before releasing parasitoids, as sticky cards attract and capture parasitoids. When scouting, look for "aphid mummies" that have circular holes on one end. These are exit holes created by emergence of adult parasitoids. Aphid parasitoids are sensitive to pest control materials. For example, direct sprays and one-day old residues of kinoprene (Enstar AQ®) are harmful to A. colemani, and direct spray applications of spinosad (Conserve®) are toxic to A. colemani.
Release parasitoids preventively on crops you know are susceptible to aphids, so that the parasitoids will be present when aphids are first noticed. Be sure to identify aphids to species before ordering parasitoids, since parasitoids attack aphid species selectively.
It is important to realize that not all commercially available natural enemies may reduce aphid populations to non-damaging levels. This is especially true when aphid numbers are abundant due to their rapid rate of development and reproduction. If high aphid populations are present in localized hot spots within the greenhouse or anticipated on hanging baskets, then apply an alternative pest control material. The use of alternative pest control materials may not disrupt already established biological control programs for aphids. Furthermore, if aphids are abundant and widespread, scattered throughout the greenhouse, then it is essential to apply an alternative pest control material to reduce the population before releasing any natural enemies. In general, alternative pest control materials do not leave toxic residues that negatively affect aphid parasitoids and/or predators.
If aphids are already abundant then it is essential to reduce their numbers before releasing any natural enemies. Do not attempt to suppress high aphid populations with predators, as this is not typically effective.
Table B-3: The common and scientific names of aphid species commonly encountered in greenhouses and the commercially available parasitoids for each aphid species
|
Aphid Common Name |
Aphid Scientific Name |
Aphidius colemani |
Aphidius ervi |
Aphidius matricariae |
Aphelinus abdominalis |
|
Cotton/melon aphid |
Aphis gossypii |
Yes |
---- |
No |
---- |
|
Foxglove aphid |
Aulacorthum solani |
No |
Yes |
---- |
Yes |
|
Potato aphid |
Macrosiphum euphorbiae |
No |
Yes |
---- |
---- |
|
Green peach aphid |
Myzus persicae |
Yes |
---- |
Yes |
---- |
Entomopathogenic Fungi
A. Beauveria bassiana: The entomopathogenic fungus, Beauveria bassiana, is commercially available for use against aphids. However, because aphids have high reproductive rates and molt rapidly, especially during the summer, repeat applications are typically required. Beauveria bassiana is most effective when aphid populations are low. This fungus may not be compatible with the convergent ladybird beetle (Hippodamia convergens), depending on the concentration of spores applied.
B. Isaria fumosoroseus: This entomopathogenic fungus is commercially available under the trade name Ancora. This fungus is most effective when the relative humidity is 75% or higher for 8 to 10 hours.
Pest Control Materials
Pest control materials (insecticides) with contact, translaminar, or systemic activity can be used to control or regulate aphid populations. Translaminar means that after application the material penetrates leaf tissues and forms a reservoir of active ingredient within the leaf. This provides extended residual activity even after spray residues dissipate. It is important to rotate insecticides with different modes of action to delay the onset of resistance. Insecticide applications must be initiated early in the cropping cycle, when plants are small. The nymphs of some aphid species reside between the scales of leaf buds or in flowers. This reduces their exposure to contact insecticides, making repeat applications necessary. A surfactant may improve coverage of wettable powder or soluble powder formulations. In some instances, insecticidal soaps and/or highly refined horticultural oils may control aphids, particularly when aphid populations are low. However, since these insecticides kill exclusively by contact and have minimal residual activity, thorough coverage of all plant parts is essential. Insect growth regulators and pyrethroid-based insecticides may also provide control or regulation of aphids. Systemic insecticides effectively control or regulate populations of aphids for extended periods of time when applied early in the cropping cycle. The same is true of the selective- feeding blockers, pymetrozine (Endeavor®), flonicamid (Aria®) and afidopyropen (Ventigra™). A number of insecticides have both translaminar and systemic properties.
Beetles
Identification, Biology and Life Cycle
Beetles are a large group of insects characterized by hardened forewings. Both adults and larvae use their chewing mouthparts to damage to a wide range of plants. Leaf-feeding beetles such as the lily leaf beetle and scarab beetles are important pests in herbaceous perennial production. Other damaging beetles include tortoise beetles, redheaded flea beetles, various spotted and striped cucumber beetles, larvae of click beetles (wireworms), and blister beetles. During weekly plant inspections, look for chewed leaves, or pinholes from flea beetle feeding.
Scarab Beetles
Scarab beetles are large, brightly colored beetles whose antennae have lamellated tips. Asiatic garden beetles, Oriental beetles, and Japanese beetles (all scarab beetles) feed on many species of herbaceous perennials, woody ornamentals and vegetables. European chafer adults are not foliage feeders; their fleshy legless larvae, known as "white grubs," feed on the roots of many plant species. Several other beetles' larvae are also called white grubs, and identification of grub species is important because the effectiveness of beneficial nematodes and other pest control materials against white grubs varies according to species. Larvae can be identified by the pattern of hairs ("rasters") on the tip of their hind end. See https://ag.umass.edu/turf/fact-sheets/white-grub-identification for images.
A. Asiatic garden beetle (Maladera castanea) adults are about 3/8 inch (0.95 cm) long and are cinnamon-brown in color. They are often found near the roots of plants. Asiatic garden beetles feed at night on aquilegia, aster, chrysanthemum, dahlia, delphinium, helianthus, heuchera, phlox, physostegia, rose, rudbeckia, salvia and zinnia. Their nighttime feeding causes C-shaped notches on the edges of leaves. During the day, adults burrow into mulch or soil/growing medium, or may be found under pots. The "white grubs" feed on the roots of grasses and flowering plants. Asiatic garden beetles overwinter as grubs in the soil/growing medium and adults emerge the following summer (mid-July to mid-August). There is one generation a year. Contact insecticides may be applied against the adults. Repeat applications may be needed.
B. Oriental beetle (Anomala orientalis) adults are about 1/2 inch (1.27 cm) long, dark brown or straw-colored, and have dark markings on their wing covers. Adults emerge from the soil/growing medium in mid-June and are present until August. They are active during the day and night. Adults do very little feeding on plant leaves. The "white grubs" feed on the roots of herbaceous perennials and woody ornamentals.
C. Japanese beetle (Popillia japonica) adults are 1/3 to 1/2 inch (8.4 to 12.6 mm) long, metallic green with coppery wing covers and white tufts of hair near the end of the abdomen. Adults feed during the day on many woody and herbaceous ornamental plants. Adults emerge from the soil/growing medium in June and July and feed for about 30 to 45 days. Eggs are laid in the soil in grassy areas and hatch into white, shaped grubs that feed on turf grass roots. Japanese beetles overwinter as grubs in the soil/growing mix below the frost line. There is one generation per year.
Scouting
Japanese beetles are extremely mobile, and once feeding begins, they emit feeding or aggregation pheromones to attract other beetles to their location. Look for feeding between leaf veins (called "skeletonization") on favored hosts. Also, check for "white grubs" in the soil or growing medium.
Cultural Control
Weed control in and around production areas helps to eliminate potential alternative food sources. Shade cloth can be used to exclude adults from hoop houses. Japanese beetles are strong fliers. Japanese beetle traps attract adult beetles, but are not recommended because they may increase feeding damage.
Biological Control
The female winsome fly (Istocheta aldrichi) is a natural parasitoid of adult Japanese beetles. Look for distinct white eggs on the thorax of adult beetles. The spring Tiphia (Tiphia vernalis) and summer Tiphia (Tiphia popilliavora) are parasitoids that attack Japanese beetle and Oriental beetle grubs. Surveys in Connecticut found that spring and summer Tiphia parasitoids are widely distributed. Surveys in Massachusetts and New Hampshire found these parasitioids in several counties as well.
Entomopathogentic nematodes are commercially available biocontrol agents that have varying levels of efficacy against scarab beetle grubs depending upon the beetle species. The recommended nematode for white grub management is Heterorhabditis bacteriophora. A recent addition to the commercially available biocontrol agents is Bacillus thuringiensis subspecies galleriae (SDS-502 strain). This biopesticide has insecticidal activity against several scarab beetle grubs and for some species, against the adult stage, too.
Pest Control Materials
Apply contact insecticides as soon as adult beetles are observed. However, many contact insecticides may be harmful to bees, predatory mites and insects. To control "white grubs", apply insecticides in grassy areas surrounding production areas.
Additional Leaf-Feeding Beetles
Lily Leaf Beetle
The lily leaf beetle (Liloceris lilii) was introduced into the U.S. in 1992, and has since spread throughout New England. Adults are 1/4 to 3/8 inches (6.3 to 9.5 mm) long and bright scarlet-red, with black legs, head and antennae. Larvae are orange, brown, or yellow. Larvae resemble fragments of soil, as they transport their excrement on their backs. The lily leaf beetle lays its eggs and completes its life cycle only on true lilies and fritillaries. Adults may feed on and cause minor damage to a few other herbaceous plants such as Solomon's seal and flowering tobacco, but they do not reproduce on these plants.
Overwintering adult beetles emerge from the soil or growing medium in early spring. Females lay up to 250 eggs over two growing seasons, on the underside of lily leaves. Larvae, which feed for approximately two weeks before entering the soil to pupate, cause most of the damage to plants. Adults emerge from pupae in 3 to 4 weeks and feed on plants until fall. Adults overwinter in soil/growing medium and plant debris.
The University of Rhode Island (URI) Biological Control Lab is researching natural enemies of the lily leaf beetle. Small parasitoids have been released throughout New England and URI researchers anticipate that these insects will disperse naturally from the release sites, eventually reducing lily leaf beetle populations.
Insect growth regulators may be effective in killing the early instar larvae. However, applications must be done early, before the larvae start covering themselves with their excrement.
Redheaded flea beetle
The redheaded flea beetle (RHFB), Systena frontalis, adults are shiny black with a red head and about 3/16 of an inch (5 mm.) long. With their enlarged femur on their hind legs, they "hop" like a flea, hence their common name. Redheaded flea beetles overwinter as eggs in the soil. Eggs hatch into white larvae with a brown head that are found in the growing media. Adults emerge from the ground in late June or early July in Connecticut. They feed on both the upper and lower leaf surfaces, skelonizating and causing holes in the leaves. Severe damage reduces marketability of the fed-upon plants. Adults may feed upon herbaceous perennials including aster, chrysanthemum, coreopsis, salvia, sedum and veronica as well as many woody ornamentals and weeds. Apply contact insecticides labeled for leaf-feeding beetles when adults are active. High volume sprays are needed to ensure thorough coverage of plant leaves and growing media.
Caterpillars
Identification, Biology and Life Cycle
Caterpillars are the larval stage of butterflies and moths. Some caterpillars are large (1 to 2 inches or 2.5 to 5.1 cm in length) whereas others, particularly when young (1st or 2nd instar), may not be visible without the aid of a 10X hand lens. Caterpillars have five or fewer pairs of prolegs (seen on the abdomen) and hooks called crochets at the tip of their prolegs.
Caterpillars that may be encountered in greenhouses include armyworms, cutworms, imported cabbage worm (Artogeia rapae), diamondback moth (Plutella xylostella), leaftiers, leafrollers (Choristoneura spp.), loopers, tobacco budworm (Helicoverpa virescens), salt marsh caterpillar (Estigmene acrea), and European corn borer (Ostrinia nubilalis). Damage is only caused by the larvae, which feed on leaves, stems, and flowers whereas adults feed on nectar or pollen.
Infestations typically begin when infested plants are introduced into a greenhouse. In addition, because adult moths such as cabbage looper (Trichoplusia ni) are active at night and are attracted to lights, females may enter greenhouses and lay eggs on plants.
Adult European corn borer females sometimes migrate from nearby cornfields and lay eggs on garden chrysanthemums and herbaceous perennials in outdoor production. The emerging larvae feed on these crops. European corn borer larvae are 3/4 to 1.0 inch (2.0 to 2.5 cm) long, and are cream colored with brown spots. Mature larvae overwinter in the stems of host plants. In New England, there are one to two generations per year. University vegetable specialists use pheromone traps to monitor European corn borer flight activity in Connecticut, Maine, Massachusetts and New Hampshire. Consult online vegetable pest messages and newsletters for this information, which may be helpful in timing insecticide applications.
Scouting
Scouting for caterpillars is important and avoids having to deal with large populations that may damage crops. Inspect plants routinely for signs of feeding damage and the presence of fecal pellets (caterpillar frass). Begin plant inspections when adults are flying. When scouting, check plants closest to greenhouse openings where adults may enter, especially areas closest to vegetable fields.
Cultural Control
Eliminate weeds that may serve as alternative hosts. Cleaning up plant debris may help remove overwintering pupae. Install insect screening over openings such as vents and sidewalls to prevent adults from entering greenhouses.
Microbial and Biological Control
A. Bacillus thuringiensis subsp. kurstaki: Spray applications of the soil-borne bacterium Bacillus thuringiensis subsp. kurstaki, also known as Btk and sold commercially as Dipel Pro DF®, Deliver®, and Javelin WG®, kill young caterpillars. This bacterium must be consumed by the caterpillar in order to be effective, and thorough coverage of all plant parts is essential. Because the bacterium is susceptible to ultraviolet light degradation, repeat applications are usually necessary. Caterpillars typically stop feeding within 24 to 48 hours after eating the bacterium, and die after 3 to 4 days.
B. Trichogramma: Parasitoids in the genus Trichogramma only attack the egg stage of various caterpillar species including diamondback moth, cabbage looper and imported cabbageworm. They do not parasitize the caterpillar stage.
Fungus Gnats
Identification, Biology and Life Cycle
Fungus gnat (Bradysia spp.) populations commonly develop in moist environments such as propagation greenhouses. The larvae are translucent to white, legless, and approximately 1/4 inch (6.3 mm) long when mature. They have a distinct black head capsule. Adults resemble mosquitoes, and are 1/8 inch (3.1 mm) long, with long legs and antennae, and their forewings display a "Y"-shaped vein pattern. Fungus gnat adults are weak fliers and may be observed resting on the growing medium surface or moving across leaves in the lower plant canopy. Adults are primarily a nuisance when present in large numbers; however, they have been implicated in carrying Botrytis spores on their bodies.
Larvae feed on fungi and decaying organic matter but they also feed on plant roots, reducing the plants' ability to take up water and nutrients. The larvae also tunnel into the crowns and stems of plants. This burrowing activity creates wounds that allow soil-borne pathogens to enter, and can kill plants. Fungus gnat larvae may also vector soil-borne pathogens such as Thielaviopsis, Pythium and Phytophthora. Fungus gnats are a common problem on geraniums, begonias, poinsettias, sedums and bulb crops, especially if the growing medium contains a high percentage of composted bark or peat moss. Younger plants are more prone to damage than older plants.
Female fungus gnats can lay up to 200 white eggs in clusters of 20 to 30 on the surface or in the crevices of moist growing media, particularly those with high organic matter content. Eggs hatch in 5 to 6 days. The larvae feed on plant roots for approximately 14 days before transitioning into pupae. Fungus gnats remain in the pupal stage for 5 to 6 days before adults emerge. Adults live approximately 10 days. The life cycle from egg to adult typically takes 21 to 28 days, depending on temperature. The presence of overlapping generations can make control or regulation difficult.
Scouting
Monitor for fungus gnat adults by placing yellow sticky cards at the base of plants, either on the growing medium surface or on the edge of flats. Inspect yellow sticky cards weekly to detect early fungus gnat infestations. Keep detailed records of the numbers of adults captured on yellow sticky cards to determine the efficacy of pest management tactics. Monitor for fungus gnat larvae by inserting potato disks or wedges into the growing medium (discussed previously). Ten potato disks may be sufficient to monitor a 10,000 ft2 greenhouse. Check disks after 48 hours, and count the number of larvae on each disk and any that are present on the growing medium surface. Replace old disks with new ones. Inspect young cuttings for signs of fungus gnat feeding and inspect root systems for fungus gnat larvae.
Cultural Control
Good sanitation practices that reduce breeding sites can prevent or minimize problems with fungus gnat populations. Avoid overwatering and allowing moisture to accumulate underneath benches. Be sure to solve drainage problems. Remove old growing medium and plant debris from inside and around the greenhouse. Inspect incoming plugs for fungus gnat larvae, if feasible, and fungus gnat adults. Fungus gnats may enter greenhouses in commercial bagged growing medium or on infested plants. Covering the growing medium with a layer of either coarse sand or diatomaceous earth does not inhibit adult emergence or prevent egg-laying by adult females. Diatomaceous earth absorbs moisture from the growing medium, resulting in the development of cracks that provide sites where larvae can pupate and females can lay eggs.
Biological Control
Several commercially available natural enemies control or regulate fungus gnat larval populations, including the soil-dwelling predatory mite, Stratiolaelaps scimitus (formerly called Hypoaspis miles); the entomopathogenic nematode, Steinernema feltiae; and the rove beetle, Dalotia coriaria (formerly called Atheta coriaria). All three natural enemies are most effective if applied before fungus gnat populations are large. In addition, the growing medium should be thoroughly moist before applying any of these natural enemies. Be sure to consult biological control suppliers for information pertaining to release rates. Below are descriptions of each of these natural enemies:
Predatory Mites
Stratiolaelaps scimitus: This soil-dwelling generalist predatory mite not only feeds on fungus gnat larvae but may also feed on thrips pupae and shore fly larvae. This mite prefers to feed on first instar fungus gnat larvae. If prey is not available, then S. scimitus feeds on plant debris and algae. It is important to initiate releases early in the growing season before fungus gnat larval populations are high. Applications can also be directed to the soil under greenhouse benches. Avoid mixing S. scimitus into growing media prior to planting because this decreases its survival. Applications must be initiated after planting and the growing medium should be moist but not saturated. S. scimitus is active when growing medium temperatures are ≥50°F (10°C). In order to evaluate this mite's effectiveness, look for reductions in numbers of fungus gnat adults on yellow sticky cards and larvae in potato disks.
Pest control materials (insecticides, miticides and fungicides), when applied as wet sprays to foliage with high-volume spray equipment, may enter the growing medium and directly or indirectly affect S. scimitus. For example, S. scimitus is negatively affected when exposed to chlorpyrifos (DuraGuard®), but pyriproxyfen (Distance®), novaluron (Pedestal®), fosetyl-aluminum (Aliette®) and mefenoxam (Subdue®) do not directly harm this predatory mite.
Predatory Beetles
Dalotia coriaria: This generalist predator feeds on fungus gnat and shore fly larvae, and supposedly thrips pupae, in the growing medium. Rove beetle adults are slender, dark brown to black, and covered with hairs. The adults are 1/8 inch (3 to 4 mm) long with very short wing covers, less than the length of their body. They fly to disperse throughout a greenhouse from original release sites. Rove beetles are strong fliers and flights often occur at night. Larvae are cream to brown in color, depending on age. Both stages inhabit cracks and crevices in the growing medium. Adults and larvae prey on fungus gnat eggs and larvae. Once established in a greenhouse, rove beetles may be present year-round, although populations may fluctuate depending on fungus gnat larval populations. Because rove beetles are generalist feeders, they may consume other natural enemies such as Stratiolaelaps scimitus. In addition, soil-dwelling predatory mites may feed on young rove beetle larvae. This interguild predation has been demonstrated in laboratory trials, but most likely does not impact their effectiveness in commercial greenhouses. Rove beetles are commercially available as both adults and larvae from many biological control suppliers. Consult your supplier about release rates.
Temperatures of 65 to 80°F (18 to 26°C) and a relative humidity of 50 to 85% are optimal for survival. However, Dalotia tolerates a wide range of conditions. Adults are nocturnal, so they are best released in the evening. Both adults and larvae are difficult to detect by scouting since they tend to hide in the cracks and crevices of growing medium. Adults can be observed on the surface of the growing medium with their abdomens raised.
One supplier sells a breeding bucket system for the rove beetles, which consists of media, beetles, and a supplier food source. These buckets are placed in shaded areas under the greenhouse benches. Growers can also make their own rearing systems. On Farm Rearing of the soil predator Dalotia coriara. MSU Organic Pest Management Laboratory. https://cerestrust.org/wp-content/uploads/MSU-Rearing-Dalotia-coriaria.pdf
The pest control materials dinotefuran (Safari®) and thiamethoxam (Flagship®) are directly toxic to rove beetle adults, whereas the insecticides Bacillus thuringiensis subsp. israelensis (Gnatrol WDG®), azadirachtin (Molt-X, Ornazin® and other generic products), diflubenzuron (Adept®), and the fungicides azoxystrobin (Heritage®), fosetyl-aluminum (Aliette®) and metalaxyl (Subdue®) are compatible with rove beetle adults. Rove beetles are also compatible with entomopathogenic nematodes.
Entomopathogenic Nematodes
Steinernema feltiae: This entomopathogenic or beneficial nematode attacks fungus gnat larvae. Infective juveniles enter the host body through natural openings such as the mouth, anus and breathing pores (spiracles) and then release a symbiotic bacterium (Xenorhabdus spp.) that kills fungus gnat larvae by dissolving their internal contents. Infected fungus gnat larvae may be opaque white to light yellow. Larvae are killed within 1 to 2 days. After the nematodes reproduce and multiply in the dead cadaver, the infective juveniles exit and search for new hosts. Nematodes are typically applied as a drench or sprench. They can also be applied through drip irrigation systems, but filters must be removed and care must be taken that the nematodes do not settle in the lines and high enough numbers are delivered through the entire length of the drip line.
Application Tips
To assess the viability of shipments prior to application, place a small quantity of the product (5 mL) in a shallow container or Petri dish and add 1 to 2 drops of tepid water. After a few minutes, look for active infective juveniles, which have a slight "S" or "J" curvature at the end of their bodies. Follow label directions to determine the appropriate number of entomopathogenic nematodes to apply.
Nematodes can also be applied through a fertilizer injector. It is essential to agitate the mixture in the stock solution with a small submersible pump so that the nematodes do not settle to the bottom of the container. Remove the filters and use a large-holed watering nozzle like a water-breaker head.
Treat crops with entomopathogenic nematodes early in the production cycle. Either apply directly to cuttings prior to sticking, or just after planting.
Repeat applications are needed to control pest populations. Growing medium temperatures must be 50 to 80°F (10 to 26°C), with optimum temperatures of 60 to 70°F (15 to 21°C). At temperatures above 88° F, (31 °C) the nematodes can die, so monitor the water and growing media temperatures during the summer months. Irrigate the growing medium before and after applying nematodes. The nematodes require moisture in order to move within the pores of the growing medium. Apply the nematodes in the evening, at dusk or on cloudy, overcast days because the nematodes are extremely sensitive to desiccation in ultraviolet light.
In general, entomopathogenic nematodes are compatible with most pest control materials, although those in the carbamate and organophosphate chemical classes may be both directly and indirectly harmful to entomopathogenic nematodes. Beneficial nematodes are also not compatible with hydrogen dioxide (ZeroTol). For more information, refer to the BASF website (http://betterplants.basf.us/) and click on "indoor greenhouses", then under product type "beneficial nematodes" click on "Nemasys beneficial nematodes", then click on "Nemasys chemical compatibility guide".
D. Miscellaneous Natural Enemies (not commercially available):
Hunter Flies: Hunter fly (Coenosia attenuata) adults can fly into unsprayed greenhouses during the growing season or come in new plantsmaterial. They can be captured in yellow sticky cards. Hunter fly adults resemble common house fly (Musca domestica) adults in appearance. They range in length from 1/8 to 1/4 inch (0.31 to 0.63 cm). Males have pale yellow legs and females have black legs. Hunter flies differ from shore flies in that their wings are uniformly clear and appear iridescent when observed in full sun perching on plant leaves. Hunter flies are nearly twice as large as shore flies.
Hunter fly adults attack and feed on fungus gnats, shore flies, whiteflies, and leafminer adults. Adult hunter flies only attack prey species that are flying. After adults capture prey in flight, they puncture the prey with their dagger-like mouthparts, and consume their prey's internal body fluids. The soil-dwelling larvae are also predaceous and feed on fungus gnat larvae and other insects that reside in the growing medium. Hunter flies establish in greenhouses by inhabiting the growing medium.
The parasitoid Synacra pauperi may be captured on yellow sticky cards, especially in unsprayed greenhouses. Females insert eggs into fungus gnat larvae. Eggs hatch into larvae that consume the internal contents of fungus gnat larvae. Parasitized fungus gnat larvae eventually pupate, and then an adult S. pauperi emerges. The population's maximum rate of increase is higher than fungus gnat larvae at 73°F (23°C).
Pest Control Materials
Insect growth regulators, microbials and other pest control materials applied to the growing medium may be effective in controlling or regulating fungus gnat larval populations. Most pest control materials do not affect eggs or pupae, so repeat applications are typically required.
The soil-borne bacterium Bacillus thuringiensis subsp. israelensis (Gnatrol WDG®) may be used before fungus gnat larval populations are high since the bacterium must be ingested in order to be effective. Applications are more effective on the young larvae (1st instar) than mature larvae (3rd and 4th instars). This material should be applied weekly until fungus gnat populations start to decline. Do not use this product if fungus gnat populations are excessive. In this case, use insect growth regulators such as pyriproxyfen (Distance®), cyromazine (Citation®), or diflubenzuron (Adept®).
Leafhoppers
Identification, Biology and Life Cycle
Leafhoppers are not common insect pests of greenhouses, although the potato leafhopper (Empoasca fabae) and the aster leafhopper (Macrosteles quadrilineatus), both pests of outdoor crops including cut flowers, woody ornamentals and herbaceous perennials, may be detected on yellow sticky cards inside greenhouses. Both leafhopper species are approximately 1/8 inch (3.0 mm) long, with slender wedge-shaped bodies. Adults hold their wings roof-like over their bodies. Nymphs resemble adults but lack wings. Leafhoppers are very active, particularly when disturbed. The adults and nymphs may walk (sideways, backwards or forward), jump or fly. Leafhoppers use their piercing-sucking mouthparts to withdraw plant fluids, causing stunting, leaf yellowing, distortion and loss of vigor. The aster leafhopper transmits the phytoplasma that causes aster yellows.
Figure B-2: Aster leafhopper. A-adult, B-front view of head, C-nymph
Figure B-3: Potato leafhopper. A & B-adults, C-egg, D-H-nymphs.
Potato leafhopper females insert eggs into the midrib or larger veins of plant leaves, or into leaf petioles or stems of astilbe, dahlia, gaura, hollyhock and lupine. Eggs hatch in 10 days. Nymphs feed on plants and undergo five molts before transitioning into adults. Adults can live up to two months. The life cycle from egg to adult may be completed in approximately four weeks. Aster leafhopper is widespread throughout the eastern United States, and is carried northward each year on wind currents. Aster leafhopper overwinters as an egg, and in southern Ontario there can be up to five generations during each growing season. Sage leafhopper (Eupteryx melissae) and mint leafhopper (Eupteryx decemnotata) have also been observed on various herbs such as rosemary, sage, catnip, mint, lavender and oregano. They are small, brightly colored and distinctly marked leafhoppers.
Scouting
Look for the fast moving adults and nymphs on the underside of leaves. Their feeding causes stippling of foliage that resembles spider mite feeding, and stunting and distortion of new growth. Potato leafhopper also injects a toxin as it feeds, so that leaves develop a V-shape, brown edge burn at the tip is known as "hopperburn" that may be mistaken for leaf scorch due to drought stress. Their white skins may be present on the foliage after shedding. Yellow sticky cards are helpful to trap the fast moving adults, and make it easier to determine the actual species.
Pest Control Materials
Control of leafhoppers with contact insecticides is difficult because they are very mobile, and new leafhoppers can enter treated areas after sprays have dried. Systemic insecticides may be applied to ornamental plants to prevent feeding damage when leafhoppers first appear.
Leafminers
Identification, Biology and Life Cycle
Leafminer species in the family Agromyzidae are common insect pests of greenhouse-grown crops. The species typically encountered in U.S. greenhouses are American serpentine leafminer (Liriomyza trifolii), serpentine leafminer (Liriomyza brassicae), pea leafminer (Liriomyza huidobrensis) and vegetable leafminer (Liriomyza sativae). Adult leafminers resemble houseflies and are 1/4 inch (6.0 mm) long with black bodies and yellow heads. In addition, yellow markings on their bodies are useful in distinguishing leafminer adults from shore fly adults. Adults may be observed walking on plant leaves and flowers. Females pierce young leaves with their ovipositor (egg-laying structure), and then they feed on the liquid that exudes from the wounded leaf tissue. Females insert eggs into leaves. Larvae that emerge from eggs are initially 1/16 inch (1.5 mm) in length and 1/13 and 1/7 inch (2.0 to 3.5 mm) long when mature. They are typically bright yellow to brown. As larvae feed within the leaf tissues, they create either serpentine or blotched mines, depending on the species. In general, leafminer females lay approximately 100 eggs during their 2 to 3 week lifespan. Eggs hatch in 5 to 6 days, and larvae feed beneath the leaf cuticle for about two weeks. The final larval instar creates an opening in the leaf and then falls to the ground. Larvae burrow into the growing medium or soil underneath benches to pupate. Leafminers require complete darkness to successfully pupate. Pupae are brown, and adults emerge within a two-week period. The duration of the life cycle depends on the host plant and temperature. For example, it takes 64 days to complete the life cycle at 59°F (15°C) but only 14 days at 95°F (35°C). There can be multiple generations each growing season.
Scouting
Inspect incoming plants for signs of leafminer activity. When visually inspecting plants, it is important to look for any egg-laying punctures, which appear as white spots on the tops of leaves. Use yellow sticky cards to monitor leafminer adult populations, especially on susceptible plant material.
Cultural Control
Avoid overfertilizing plants, particularly with nitrogen. Overfertilized plants are more attractive to adult females for egg-laying. Remove weeds, plant debris and infested leaves before leafminers pupate. Hopper tape could be used to trap out adults, if no parasitic wasps are being released.
Biological Control
Commercial natural enemies for leafminers include two parasitoids, Diglyphus isaea and Dacnusa sibirica.
A. Diglyphus isaea is a small (1/25 to 1/11 inch or 1.0-2.3 mm long) ectoparasitoid; females paralyze the host or prey before laying one or more eggs adjacent to leafminer larvae. Adult females attack second instar leafminer larvae within leaf mines. They are best used in spring and summer with high temperatures (68°F or 20°C) and when pest populations are high.
B. Dacnusa siberia is 1/11 to 1/9 inch (2.5 to 3.0 mm) long with extended antennae. It is an endoparasitoid (females lay eggs directly into leafminer larvae) that uses odors emitted from leafminer frass to locate larvae within damaged plant tissue. Dacnus is better adapted to lower temperatures (60° F or 15° C), short days and lower light levels than Diglyphus.
Both of these parasitoids are most effective in controlling or regulating leafminer populations in long-term crops such as cut flowers and stock plants, and they should be used preventively to control or regulate low leafminer populations. When scouting, look for short mines with the dead leafminer larvae inside the mines. Avoid applying broad spectrum pest control materials in the organophosphate, carbamate and pyrethroid chemical classes since many of these materials leave residues that may persist and kill both parasitoids for several weeks. Release the parasitic wasps in the morning or evening. Remove yellow sticky cards before making releases, as parasitoids are attracted to them. Replace the cards 3-4 days after applying the parasitoids. Diglyphus isaea has proven to be effective in controlling or regulating leafminers in greenhouse-grown chrysanthemums and Transvaal daisy (Gerbera jamesonii).
Pest Control Materials
Using insecticides to control or regulate leafminer populations may be difficult because several species have developed resistance to a number of commonly used insecticides. Applications of pyrethroid-based insecticides may be required every 3-4 days to kill adults as they emerge from pupae in the growing medium or soil. Apply in the morning, when females are actively laying eggs, as wet sprays may disrupt their ability to deposit eggs in leaf tissue. Several pyrethroid-based insecticides have repellent properties that may deter adult females from laying eggs, thus minimizing damage to plant leaves. Insecticides with translaminar properties, including several insect growth regulators, are effective in killing larvae within leaf mines.
Mealybugs
Identification, Biology and Life Cycle
Mealybugs are typically a problem on long-term crops such as cut flowers, orchids, and foliage plants. Species encountered in greenhouses include the citrus mealybug (Planococcus citri), longtailed mealybug (Pseudococcus longispinus) and obscure mealybug (Pseudococcus viburni). Longtailed mealybug can be a major problem on conservatory plants such as cycads, orchids, palms and ferns. It is easy to identify due to the presence of long waxy filaments that protrude from the end of its abdomen. Obscure mealybug also has waxy filaments but they are much shorter than those of longtailed mealybug. Citrus mealybug lacks any waxy filaments on the body and has a gray stripe that extends the length of the body. Mealybugs that have been introduced into the U.S. and which may also be present in greenhouses include the pink hibiscus mealybug (Maconellicoccus hirsutus), madeira mealybug (Phenacoccus madeirensis), and the Mexican mealybug (Phenacoccus gossypii).
Mealybugs usually enter a greenhouse on infested plant material. Mealybugs use their piercing-sucking mouthparts to withdraw plant fluids. Both nymphs (referred to as crawlers) and adults feed on plants and cause stunting, leaf yellowing and distortion of plant parts. While feeding, mealybugs may inject toxic saliva into plant tissues, and excrete a copious amount of honeydew (clear, sticky liquid) that serves as an excellent growing medium for black sooty mold fungi. The presence of black sooty mold fungi inhibits plants' ability to manufacture food via photosynthesis, and detracts from the plants' aesthetic appearance.
Mealybugs are soft-bodied, segmented, oval-shaped insects. Adult females are 1/20 to 1/5 inch (1.0 to 5.0 mm) long and are usually covered with a white, powdery, waxy material. A fringe of waxy filaments may be present on the margin of their body. Mealybugs retain their legs throughout development, allowing them to move from plant to plant within a greenhouse. Newly emerged nymphs or crawlers mature in 6 to 9 weeks. Some mealybug species, like the longtailed mealybug, give birth to live offspring. Other species, such as citrus and obscure mealybug, lay masses of up to 100 yellow-to-orange eggs in a white cottony sac. Eggs hatch in 5 to10 days, but unhatched eggs or young nymphs may remain inside the cottony sac if environmental conditions such as temperature and relative humidity are not favorable. Adult males of most mealybug species are small winged insects that lack functional mouthparts. Their primary role is to fertilize females.

Figure B-4: Adult mealybug
Scouting
Early detection and isolation of infested plants is important to avoid mealybug outbreaks. Routine visual inspections of susceptible plants make it easier to deal with mealybugs with either pest control materials or natural enemies. Mealybugs feed on a wide range of plant hosts including coleus, croton, dracaena, English ivy, fuchsia, gardenia, hibiscus, mandevilla, stephanotis, palms, orchids, cacti and succulents. Mealybugs are usually located on leaf undersides, petiole and leaf junctions, and near the base of plants. They may also be present on the inside of container lips and in container drainage holes.
Root-feeding mealybugs (Rhizoecus spp.) appear as masses of wax, and may occasionally be detected on the roots of wilting plants. These mealybugs, which are not as active as mealybugs that feed above ground, are typically covered with a fine, powdery, wax-like material. Root mealybugs do not have the marginal filaments that are typical of other mealybugs. Nymphs are covered by a white waxy material, and are located in the crevices of growing media or in excavated chambers on the outer edge of the root ball. Root mealybug feeding causes stunting and leaf yellowing. Damage is usually noticeable when high populations are present. Once introduced into a greenhouse, root mealybugs may spread to other plants in water that drains from containers, in growing medium or plant debris, or on equipment. Root mealybugs may also infest adjacent plants by crawling through drainage holes of containers. Discard all infested plants and debris from a greenhouse where root mealybugs are present. Disinfect/disinfest contaminated containers and benches before reusing them.
Cultural Control
Inspect incoming plants for signs of mealybugs. Start with clean plant material. Do not carry over stock plants or "pet plants" that may be mealybug-infested. Immediately dispose of severely infested plants. Power wash greenhouse benches between crops. Do not re-use pots.
Biological Control
Parasitoids may suppress or regulate mealybugs in conservatories and where plants are maintained for extended periods. Identify mealybugs to species before releasing any natural enemies. Anagyrus pseudococci, a parasitoid, attacks the citrus mealybug, parasitizing both the second and third larval stages and adults.
In general, mealybug predators are less efficacious than mealybug parasitoids. The predatory ladybird beetle Cryptolaemus montrouzieri, also called the "mealybug destroyer," may be used to control or regulate citrus mealybug populations. Both adults and larvae prey on all stages of mealybugs. Mealybug destroyer larvae resemble mealybugs but are covered with waxy appendages and are more mobile than mealybugs. The mealybug destroyer is more effective from spring through fall than in winter. This predatory beetle prefers temperatures above 60°F (15°C). Release in the evening. Place in distribution boxes in the crop canopy. Consult your supplier for recommended release rates. Pest control materials such as dinotefuran (Safari®) and acetamiprid (TriStar®) have been shown to directly harm the mealybug destroyer whereas pyriproxyfen (Distance®) and flonicamid (Aria®) are not directly harmful.
The host specific parasitic wasp (Anagyrus pseudocci) parasitizes citrus mealybug larvae.
Pest Control Materials
It is difficult to control or regulate mealybug populations with pest control materials (insecticides) due to their waxy coating, which reduces contact and penetration of sprays. The young nymphs or crawlers, however, don't have a waxy covering so they are susceptible to spray applications of many insecticides. Systemic insecticides may not work as well applied as a drench compared to a spray. Contact insecticides such as insect growth regulators, insecticidal soap and horticultural oil effectively kill young nymphs. However, as eggs hatch throughout the growing season, repeat applications are required. Application frequency depends on the residual activity of insecticides used, varying from 1 to 3 weeks. Thorough coverage of all plant parts is essential. The use of a spreader-sticker may improve coverage and penetration, but may also increase the risk of phytotoxicity (plant injury). Insecticidal soaps and horticultural oils effectively kill eggs, nymphs and young adults. Systemic insecticide drenches applied to the growing medium may effectively control or regulate root mealybug populations.
Mites
Mites are more closely related to spiders and ticks than to insects. Mites, in general, use their piercing-sucking mouthparts (stylets) to puncture plant cells and then withdraw cell contents. All mites are wingless, and their head and thorax are fused together. The body is compact, and oval to oblong in shape. Mites are small (<1/16 inch or 1.6 mm long) and typically difficult to detect without the aid of magnification such as a 10X hand lens. Some species are so small that they can only be observed with a dissecting microscope. Gender, in many mite species, depends on fertilization of the females by males. For example, unfertilized eggs produce males whereas fertilized eggs produce females. Newly hatched larvae only have three pairs of legs. The larval stage is followed by two nymphal stages (deutonymph and protonymph) and then the adult. Both nymphs and adults have four pairs of legs. Several species of tetranychid and tarsonemid mites can be severe pests of many greenhouse-grown crops. The common mites encountered in greenhouses include the bulb mite, broad mite, cyclamen mite, Lewis mite and twospotted spider mite.
Bulb Mites
Identification, Biology and Life Cycle
Bulb mites (Rhizoglyphus species) infest bulb crops such as amaryllis, crocus, freesia, gladiolus, hyacinth, lily, narcissus and tulip. The two most common species are Rhizoglyphus echinopus and R. robini. Bulb mites have a short life cycle and high reproductive potential. The life cycle consists of an egg, larva, protonymph, deutonymph, tritonymph and adult. Bulb mites are 1/50 to 1/25 inch (0.5 to 0.9 mm) long, smooth, and shiny white to translucent with two brown spots on the body, with short red-orange legs. Each female bulb mite lays up to 100 white eggs during her life. The life cycle takes approximately 40 days to complete. However, this depends on relative humidity, plant type and temperature. For example, at 77°F (25°C), the life cycle takes 12 days.
Scouting
Visible signs of damage are typically not apparent until bulb mite populations are extensive. In general, bulb mites are secondary arthropod pests commonly associated with decaying organic matter as a result of damage caused by fungus gnat larvae and soil-borne root pathogens. However, bulb mites do feed on roots and below-ground structures of certain plants. They infest bulbs and corms by penetrating the basal plate or outer skin layers.
Cultural Control
Bulb mites that establish in the inner bulb layers are difficult to control or regulate. Store bulbs in cool temperature and low relative humidity to prevent or minimize problems with diseases, and thus prevent the build-up of bulb mites. Bulb mite populations may be controlled or regulated by immersing infested plants in 110°F (43°C) water for 30 minutes. However, this is a short-term remedy, and may directly or indirectly damage some bulb crops.
Pest Control Materials
Currently, no miticides are labeled to control or regulate bulb mite populations in greenhouses.
Broad Mites
Identification, Biology and Life Cycle
Broad mite (Polyphagotarsonemus latus) is a tarsonemid mite that is often misidentified as cyclamen mite. Broad mite may be distinguished from cyclamen mite by the fact that broad mite eggs are covered with conspicuous white protrusions, while cyclamen mite eggs are smooth. Broad mite adults and larvae are smaller and more active on host plants than cyclamen mite adults and larvae. Broad mites favor temperatures of 70 to 80°F (21 to 26°C) and relative humidity of 80 to 90%. Their life cycle may be completed in about one week at 70°F (21°C).
Figure B-5: Broad mite. A-adult female, B-male, C-egg, D-nymph
Scouting
Broad mite, like cyclamen mite, feeds on a wide range of host plants including African violet, ageratum, azalea, begonia, chrysanthemum, dahlia, exacum, Transvaal daisy (gerbera), impatiens, English ivy, lysimachia, New Guinea impatiens, petunia (vegetatively propagated), pepper, salvia, snapdragon, verbena and zinnia. Broad mites typically feed on the lower surface of young leaves. Broad mite feeding prevents normal leaf expansion and causes a downward "puckering" along the leaf edges. Heavily infested leaves may appear purple. Terminal buds may also be killed, particularly if high broad mite populations are present. Flowers and/or buds may be distorted and fail to open. Damage often occurs suddenly on the newest growth and often only one or two plant species are infected. Broad mite feeding damage may resemble herbicide injury, certain nutrient deficiencies, or several physiological disorders. Broad mites may be spread among plants via air currents, leaves of plants in contact with each other, by workers handling infested plants and then touching uninfested plants, and on whitefly adults. Dispose of all plants displaying damage symptoms from broad mite feeding immediately. Furthermore, any plants adjacent to infested plants should also be disposed of promptly.
Pest Control Materials and Biological Control
The pest management options associated with the use of both pest control materials and natural enemies are the same for broad mite and cyclamen mite (described under cyclamen mite below).
Cyclamen Mites
Identification, Biology and Life Cycle
Cyclamen mite (Phytonemus pallidus) is a very small (1/100 inch or 0.25 mm long) tarsonemid mite that feeds on a wide range of greenhouse crops. Because of their small size, greenhouse growers initially notice their damage rather than the mites themselves. Cyclamen mites favor cool temperatures (60°F or 15°C) and high relative humidity (80 to 90%). Infestations in the Northeast are common in fall and winter.
Cyclamen mites are semitransparent and light green when viewed under a dissecting microscope. Females have an elliptical shape with two pairs of stout front legs and two pairs of slender hind legs. Each hind leg has two bristles; the one on the inner side is longer. Males are square in shape with claws on the hind pair of legs instead of bristles. Both males and females move very slowly. Females live up to one month and lay up to 16 eggs. Eggs are oval and glossy white, and are typically deposited in buds or in folds of young leaves. White larvae emerge from eggs in approximately three days. The life cycle from egg to adult takes 1 to 3 weeks.
Scouting
Cyclamen mites feed in terminal buds and on young expanding leaves. Damage symptoms include bud abortion, inward curling of leaves or leaflets and/or distorted and hardened leaves. Flower buds may abort or emerging petals may be deformed. Send plants exhibiting suspected cyclamen mite damage to a diagnostic clinic. Plants susceptible to cyclamen mite include African violet, azalea, begonia, carnation, chrysanthemum, cyclamen, dahlia, delphinium, exacum, fuchsia, geranium, Transvaal daisy (gerbera), gloxinia, hydrangea, impatiens, English ivy, kalanchoe, lamium, larkspur, petunia, rosemary, snapdragon, verbena, viola and zinnia.
Cultural Control
Cyclamen mites can spread throughout a crop via air currents, leaves of plants in contact with each other, and by workers handling infested plants and then touching uninfested plants. Immediately dispose of all plants displaying damage symptoms from cyclamen mite feeding, and any plants adjacent to those infested plants.
Biological Control
Predatory mites—Neoseiulus (Amblyseius) cucumeris, Amblyseius andersonii, Amblyseius swirskii, and Neoseiulus (Amblyseius) californicus—control or regulate cyclamen mites on some crops. However, these predatory mites must be released before cyclamen mite populations are high and plant damage is obvious. Consult your biological control supplier for more information about recommended predatory mites and release rates.
Pest Control Materials
Cyclamen mites are hard to control or regulate because they feed in secluded locations on plants, such as the meristematic tissues, where they avoid exposure to spray applications of contact pest control materials. Therefore, translaminar miticides are recommended to regulate cyclamen mite populations. Thorough coverage is essential in order to get enough active ingredient into the plant tissues where cyclamen mites feed. Two or more applications are usually required to obtain control or regulation of cyclamen mite populations, thus preventing them from spreading to other crops. Cyclamen mites may also be controlled or regulated by immersing infested plants in 110°F (43°C) water for 30 minutes. This is a short-term remedy, and may directly or indirectly damage some crops.
Twospotted Spider Mites
Identification, Biology and Life Cycle
The twospotted spider mite (TSM), Tetranychus urticae, the predominant mite species in greenhouses, feeds on a wide-range of crops including herbs, herbaceous annuals and perennials and vegetable bedding plants. TSM feeds within leaf cells, damaging the palisade and spongy mesophyll cells and chloroplasts, thus reducing chlorophyll and moisture content and the plant's ability to photosynthesize. This may result in characteristic symptoms such as leaf bleaching, yellow stippling and bronzing. Even low populations may cause stippling on mature leaves. Large populations of TSM may cause leaf yellowing and distortion of terminal buds and flowers. In addition, there may be abundant webbing on leaves, petioles and stems. TSM populations increase rapidly when temperatures exceed 80°F (26°C) and relative humidity is 20 to 40%. The life cycle from egg to adult can be completed in seven days at temperatures >85°F (29°C).
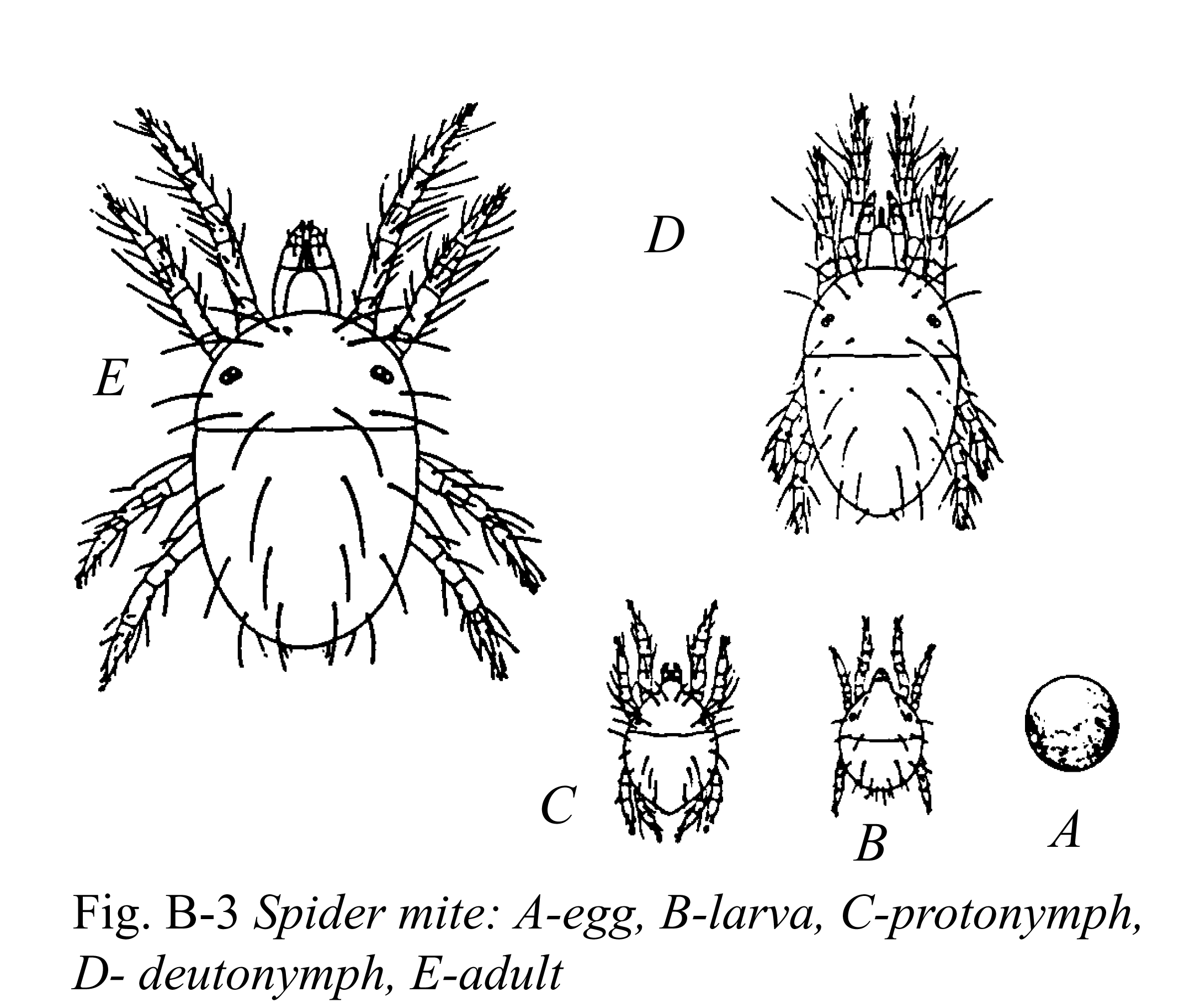
Figure B-6: Spider mite. A-egg, B-larva, C-protonymph, D-deutonymph, E-adult
Adult females are oval, approximately 1/50 inch (0.5 mm) long and green to orange in color with two dark spots on both sides of the abdomen. Adult females lay up to 12 eggs per day and can up to 100 eggs during their 3- to 4-week lifespan. Eggs are globular and amber red when viewed with a 10X hand lens. Eggs hatch in approximately three days, and young mite larvae immediately begin feeding. After two nymphal stages (deutonymph and protonymph), mites become adults. Females, which do not have to mate to reproduce, begin laying eggs within 1 to 3 days.
Scouting
Inspect plants weekly for signs of TSM feeding injury. Susceptible plants include angel's trumpet, bee balm, butterfly bush, cordyline, dahlia, delphinium, dracaena, eggplant, Transvaal daisy (gerbera), ivy geranium, mandevilla and other tropical plants, New Guinea impatiens, phlox, marigold, salvia, scabiosa, scaevola, thunbergia and verbena. Look for mites on the underside of mature leaves, especially along the midvein and on lower leaves. When scouting, check for the presence of live TSM and their round eggs. Initial infestations commonly occur in warm and dry locations such as near steam pipes, furnaces or heaters, or hanging baskets positioned overhead with drip irrigation.
Cultural Control
Remove weeds in and around greenhouses, as they provide refuge for TSM. Dispose of old plant material including "pet plants" which may harbor populations of TSM. Proper crop irrigation decreases susceptibility to TSM. Avoid overfertilizing plants, especially with soluble nitrogen fertilizers, because this promotes the production of succulent growth that is easier for TSM to feed on. Overfertilized plants contain higher concentrations of amino acids that are essential food sources for TSM, and may enhance development and reproduction of TSM females. Drip irrigation is a more efficient way to water plants, but occasional overhead irrigation can wash some TSM off plants.
Routine plant inspection helps avoid dealing with TSM populations with all life stages present simultaneously. This in turn maximizes the effectiveness (based on percent mortality) of any miticide applications. Furthermore, this may reduce the number of applications needed, which is important because TSM populations can easily develop resistance to miticides, making control or regulation very difficult. It is essential to rotate miticides with different modes of action in order to delay the onset of resistance.
Biological Control
Commercially available predatory mites that may control or regulate TSM populations include Phytoseiulus persimilis, Galendromus occidentalis, Neoseiulus (Amblyseius) californicus, Amblyseius (Neoseiulus) fallacis, Amblyseius andersonii, and Mesoseiulus longipes. Each species requires and is adapted to different temperatures and relative humidity). For example, G. occidentalis, M. longipes, N. fallacis, and N. californicus tolerate warmer conditions (>85°F or 29°C) and lower relative humidity (30 to 40%) than P. persimilis. Furthermore, these predatory mites generally persist at low populations of TSM.
The effectiveness of predatory mites depends on TSM population levels and alternate food sources. For example, P. persimilis only feeds on TSM, while the other predatory mite species either feed on alternative hosts or prey, or on flower pollen.
Additional commercially available predators of TSM include the predatory midge, Feltiella acarisuga, and the ladybird beetle, Stethorus punctillum. Consult your supplier for information on release rates.
Below are descriptions of the predators commercially available for control or regulation of TSM populations. Keep mite predators cool prior to release, but do not store them in the refrigerator. Gently mist plants before sprinkling the predatory mites on the lower leaves.
Mite Predators
A. Phytoseiulus persimilis: This is the most effective predatory mite for control or regulation of TSM populations. Phytoseiulus persimilis spreads among crops to locate TSM colonies using odors emitted from infested plants. This is a specialist predatory mite that only uses TSM as a food source, feeding on all life stages (eggs, larvae, nymphs and adults). So, be sure to order them from producers that can insure overnight shipping and delivery. P. persimilis adults are bright red, pear-shaped with long legs, and are larger and more active than TSM. Adult females lay eggs that are approximately 2 to 3 times as large as TSM female eggs. Both the adults and nymphs actively search plants for TSM. Initiate releases early when TSM populations are low or when TSM are first detected. Two to three applications, one week apart, may be required. Make releases near TSM infestations and concentrate releases near localized hot spots. The temperature must be around 68°F (20°C) with 75% relative humidity in order for this predatory mite to be effective. Plants need to be touching for the predatory mites to disperse through the crop. Gently sprinkle predatory mites on the lower leaves. When temperatures exceed 86°F (30°C), P. persimilis cannot keep up with the reproductive capacity of TSM. When relative humidity is <60%, predatory mite female eggs desiccate or fail to hatch. When scouting, check for the presence of live TSM and their round eggs. Also look for the predatory mites, which are pear-shaped and move quickly when disturbed. You can shake plants or plant parts over a white sheet of paper (8.5 x 11 inches or 22 x 28 cm) and observe the predatory mites. P. persimilis eggs are elliptical, shaped more like a "football" than the round TSM eggs. Look for eggs to be sure that the beneficial predatory mites are reproducing. Look for deflated spider mite eggs that have been sucked dry by the predatory mites.
Pest control materials that have been demonstrated to be compatible with P. persimilis include spinosad (Conserve®), pymetrozine (Endeavor®) and clofentezine (Ovation®). However, bifenazate (Floramite SC®), spiromesifen (Savate®) and chlorfenapyr (Pylon®) may be harmful to P. persimilis.
B. Neoseiulus (Amblyseius) californicus: This selective predatory mite has a broader host or prey range than P. persimilis, and survives longer in the absence of prey by feeding on other plant-feeding mites and thrips. They may even feed on mold and nectar. Neoseiulus californicus is the appropriate choice under high temperatures and relative humidity, and when TSM populations are moderate to low. N. californicus is most active at temperatures of 46 to 90°F (8 to 32°C) and relative humidity of 40 to 80%. However, when TSM populations are low, populations of N. californicus tend to decline faster than P. persimilis. It is available in bulk or as mini-sachets. N. californicus is compatible with the following pest control materials: bifenazate (Floramite SC®), chlorfenapyr (Pylon®), spiromesifen (Savate) and spinosad (Conserve®).
C. Amblyseius andersonii: This predatory mite may feed on TSM, broad mites, cyclamen mites, and eriophyid mites. It may also survive on thrips and fungal spores in the absence of mites. Amblyseius andersonii can be released in the presence of low populations of TSM. If hot spots develop, P. persimilis can be used in conjunction with this species. A. andersonii can be applied to both greenhouse and outdoor crops and is active at temperatures of 42 to 104°F (6 to 40°C). This predatory mite is available in bulk and as mini-sachets.
D. Feltiella acarisuga: This small (1/16 inch or 2.0 mm) predatory midge feeds on TSM. Adults live up to three days and are active at night, resting during the day on leaf undersides. Females lay orange-to-red eggs among TSM colonies. Eggs hatch in 3 to 5 days. The larvae are the only predaceous stage, feeding on all life stages (eggs, larvae, nymphs and adults) of TSM. After 5 to 7 days, larvae transition into a white, velutinous pupal stage on leaf undersides. Adults emerge from pupae, and although they do not feed, they can fly, allowing them to locate TSM populations on hanging baskets or other locations within the greenhouse that are not accessible to predatory mites. Optimum environmental conditions for survival include temperatures of 68°F to 80°F (20°C to 26°C), and 80% relative humidity. Extended periods of relative humidity below 60% may reduce survival and reproduction. Feltiella acarisuga is active year-round, and does not have a winter resting stage. This predatory midge is shipped as pupae, and adults emerge soon after arrival. Release late at night or early in the morning. When scouting, look for the nearly white pupal cases near the midrib on leaf undersides and for the bright orange larvae.
E. Predatory Mites Adapted to Outdoor Growing Conditions: Mesoseiulus longipes is similar to P. persimilis in activity, but can tolerate warmer temperatures (up to 90°F (32°C) and relative humidity as low as 40%. The predatory mite Neoseiulus fallacis can survive low temperatures and prey availability, and is resistant to some pest control materials. It is useful for controlling or regulating TSM populations in outdoor situations. Similar to P. persimilis, N. fallacis rapidly reduces extensive TSM outbreaks. Galendromus occidentalis, another predatory mite, tolerates a wide-range of temperatures and relative humidity, and is well adapted to outdoor conditions.
F. Stethorus punctillum: This small (0.03 to 0.05 inch, or 1.0 to 1.5 mm long) black predatory ladybird beetle feeds on all life stages of TSM. Adults can fly, allowing them to locate TSM colonies that are not accessible to predatory mites.
If pest control materials with long-residual activity are applied, avoid releasing predatory mites for about four weeks since any residues may kill the predatory mites. However, any direct and indirect effects may vary depending on the pest control material. For example, spinosad (Conserve®) is compatible with most TSM predators, but residues of abamectin (Avid®) may be toxic for up to 14 days after application.
Pest Control Materials
Contact or translaminar miticides may be used to control or regulate TSM populations. Translaminar means that after application the material penetrates leaf tissues and forms a reservoir of active ingredient within the leaf, providing extended residual activity even after spray residues dissipate. Miticides with translaminar properties include abamectin (Avid® and many generic abamectin products), etoxazole (TetraSan®), chlorfenapyr (Pylon®) and spiromesifen (Savate ®). Read the label before making an application to determine the life stages (e.g., egg, larva, nymph, and/or adult) that are most affected by the miticide as well as plant safety precautions. Spray infested plants, then mark several plants and use a 10X hand lens to detect the presence of live and dead TSM. Repeat applications every 5 to 7 days, as most miticides are not effective against the egg stage. Thorough coverage of both the lower and upper leaf surfaces is critical when applying miticides with contact activity. Insecticidal soaps and horticultural oils are also effective against TSM; however, certain plants may be sensitive to both pest control materials. Consult the label to determine which plants to avoid spraying. Mite growth regulators such as etoxazole (TetraSan®) must be applied before populations of TSM are extensive. This mite growth regulator may be tank-mixed with a miticide that is active on adults. Routine plant inspections help to avoid dealing with TSM populations in which all life stages are present simultaneously. This in turn helps maximize the effectiveness (based on percent mortality) of any miticide applications, and may also reduce the number of applications needed, which is important because TSM populations easily develop resistance to miticides, making control or regulation very difficult. It is essential to rotate miticides with different modes of action, to delay onset of resistance.
Lewis Mites
Lewis mite (Eotetranychus lewisi), which is related to twospotted spider mite (TSM), feeds primarily on poinsettia (Euphorbia pulcherrima). It produces less webbing than TSM. Lewis mites are small (1/16 inch or 1.6 mm long), slender and straw-colored. Adults have several tiny spots along each side of the abdomen whereas TSM adults have two distinct markings. Leaves fed upon by Lewis mite have a flecked or stippled appearance. Lewis mite feeding injury resembles nutrient deficiencies. Immediately discard any plants infested with Lewis mites plus adjacent plants. Damage is usually evident from August through October. Predatory mites recommended for control or regulation of TSM (discussed above) may be used against Lewis mites on most crops. Implement releases before high Lewis mite populations are present. Miticides recommended for control or regulation of TSM populations may not be effective on Lewis mite. Read the labels and rotate miticides with different modes of action in order to delay the onset of resistance.
Plant Bugs
Identification, Biology and Life Cycle
Tarnished plant bug, Lygus lineolaris, is the most common plant bug in greenhouses in the Northeast. Tarnished plant bugs feed on many weeds and outdoor plants, migrating into greenhouses through doors, sidewalls and louver vents. They are oblong, tapering toward the end of the abdomen, and bronze. This color differentiates them from the more brightly colored four-lined plant bug (Poecilocapsus lineatus) that has distinct yellow and black lines or markings along the back of its abdomen. The four-lined plant bug is not common in greenhouses. Tarnished plant bugs have small triangular heads and prominent eyes. Adults are winged and very active, especially when disturbed. Females insert eggs into flower petals, plant stems, leaf petioles, or along leaf midribs. This causes swelling of plant tissues. Eggs are elongate and slightly curved with one end embedded in plant tissue. Eggs hatch in 7 to 10 days. Newly emerged nymphs are wingless, 1/10 inch (2.0 mm) long, yellow green, with long legs. They may be misidentified as aphids. Tarnished plant bug undergoes five nymphal stages, with the last two resembling adults. The life cycle may be completed in 3 to 4 weeks.
Figure B-7: Tarnished plant bug. A-adult, B-E-nymphs
Tarnished plant bugs overwinter as adults under weeds or debris such as old plants and growing medium debris near greenhouse openings. Both adults and nymphs use their piercing-sucking mouthparts to feed on plant fluids while injecting toxic saliva that kills plant tissues.
Scouting
Gently tap foliage over a sheet of white paper to look for nymphs and adults. White sticky traps are also available to monitor for adults. Tarnished plant bug feeding causes leaf yellowing and terminal growth distortion. Leaves may look ragged or discolored, and flowers fail to develop or flower buds may abort.
Cultural Control
Remove weeds and plant debris within and around the greenhouse perimeter to minimize potential problems with this insect pest.
Pest Control Materials
Contact insecticides may be used to kill adults and/or nymphs feeding on plants in the greenhouse.
Sawflies
Identification, Biology and Life Cycle
Some species of sawflies are pests of herbaceous perennials. Although sawflies may be confused with caterpillars, it is important to distinguish between the two, because their management strategies differ. Caterpillars generally have two to five pairs of fleshy prolegs on their abdomen. Their prolegs have hooks or spines at the tip, which help them attach to plants. Bacillus thuringiensis subsp. kurstaki, or Btk, is specific to caterpillars and is not effective against sawflies.
Sawflies, in contrast, have six or more pairs of fleshy prolegs on their abdomen. Adults are thick-waisted, non-stinging wasps with slender antennae. Sawfly larvae feed together in groups, and can cause extensive damage. The pale green columbine sawfly (Pristophora aquiligae) can defoliate columbine in the spring. Hollyhock sawfly (Neoptilia malvacearum) feeds on hollyhock. Hibiscus sawfly (Atomacera decepta) can be very damaging to susceptible hibiscus species. All sawfly larvae feed in groups on leaf tissue but not veins, giving leaves a lacy appearance.
Scouting
Look for the thick-waisted bee-like adults in the early morning, resting on plant leaves. The early instar larvae feed on the underside of the leaves.
Pest Control Materials
Spinosad (Conserve) or a pyrethroid-based insecticide may be effective against sawflies.
Scales
Identification, Biology and Life Cycle
Armored (Diaspididae) and soft (Coccidae) scales are frequently seen on ornamental plants. Adult females are wingless, usually legless, and resemble sacs. Only two stages are mobile: the first instar nymph (crawler) and adult male. The first instar nymphs emerge from eggs and move about on plants searching for a suitable place to feed. Later nymphal stages and adult females are immobile. Adult males are winged, and live only a few days. They lack functional mouthparts and do not feed. Their primary role is to fertilize females. Scales damage plants by using their piercing-sucking mouthparts to withdraw plant fluids and simultaneously inject toxic saliva. Contact your Extension entomologist for identification of armored and soft scales. Specimens may be shipped live or prepared in 70% isopropyl alcohol (rubbing alcohol).
Armored or Hard Scales
The biology and life cycle of armored or hard scales vary among species. Generally, first instar nymphs or crawlers have legs and antennae, and move around on plant parts without feeding for up to two days. After locating a suitable site on a host plant, they settle down, insert their piercing-sucking mouthparts into plant tissues, and withdraw plant fluids. Legs and antennae fall off after the first molt, and a waxy covering is secreted to protect the body, which increases in size after each subsequent molt. Females, depending on species, lay eggs or give birth to live offspring under the covering. Several generations may occur per year. Males undergo a pupal stage and then emerge as winged adults. The visible "scale" is not the actual body of the insect but rather a layered integument called the "cover" or "testa." The female body is not attached to this layered covering, which consists of wax and molted skins from earlier instars, and may vary depending on the species being circular, elliptical, oyster shell-like, smooth or rough. Scale color varies by species. In general, the male scale cover is more elongate than the female cover. However, males do not exist for some armored scale species in which females give birth to live offspring. Some common armored scale species that may be seen in greenhouses and interiorscapes include Boisduval's scale (Diaspis boisduvalii), oleander scale (Aspidiotus nerii), San Jose scale (Quadraspidiotus perniciosus), Florida red scale (Chrysomphalus aonidum), fern scale (Pinnaspis aspidistrae), greedy scale (Hemiberlesia rapax) and purple scale (Lepidosaphes beckii). Armored scales typically feed on woody ornamentals, not herbaceous plants. Also, armored scales, depending on species, typically feed on only one or two host plant types.
Soft or Bark Scales
Soft or bark scale females, unlike armored scale females, are not hidden beneath an integument as their bodies are covered primarily by wax, and the actual living scale is easy to distinguish. Soft scales are flattened, and oval or globular. In contrast to armored scales, soft scales excrete large quantities of honeydew, which serves as a growing medium for black sooty mold. Honeydew may also attract ants. Eggs are laid in cottony sacs protruding from the end of the female body.
Figure B-8: Brown soft scale. A-mature female, B-side view of female, C-crawler
Females may produce eggs continuously over several months. Some soft scale species, although stationary, retain their legs and antennae as adults. Males may be winged or wingless. Soft scale species that may feed on plants grown in greenhouses or interiorscapes include black scale (Saissetia oleae), brown soft scale (Coccus hesperidum), hemispherical scale (Saissetia coffeae), and nigra scale (Parasaissetra nigra). Unlike armored scales, soft scales feed on a wide range of woody and herbaceous plants.
Figure B-9: Hemispherical scale. A-top view, B-side view, C-dead adult with eggs, D-crawler
Scouting
Inspect foliage and stems for the presence of scale covers. If the scale cover is a hollow shell, and you do not see any legs moving, it is a dead scale from a previous generation. Honeydew and black sooty mold on plant leaves indicate the presence of soft scales. Armored scales do not product honeydew.
Cultural Control
Inspect incoming plants for scale insects. Remove heavily infested plants or plant parts when crawlers are not active. Avoid over-fertilizing plants, particularly with nitrogen, as this encourages the development and reproduction of scale insects.
Biological Control
Biological control of armored and soft scales may be difficult due to the wide-range of scale species that may occur simultaneously. Currently, the number of commercially available natural enemies for control or regulation of both armored and soft scale populations is limited. Commercially available predators that control or regulate armored and soft scale populations include Rhyzobius (=Lindorus) lophanthae. Initiate releases early, before scale populations rise to damaging levels. Multiple releases of natural enemies may be required. Consult your supplier for information on release rates.
Pest Control Materials
Insecticides such as insecticidal soap and horticultural oils are very effective in controlling or regulating scale populations when used properly. The best way to control or regulate both armored and soft scale populations is to make applications when nymphs or crawlers are present, because they are the most susceptible life stages. However, repeat applications are typically required. Horticultural oils may kill eggs and even older life stages via suffocation by coating the breathing spores (spiracles). However, the later stages of armored scales, such as the mature females, are less susceptible since their outer covering prevents penetration of insecticide through the cuticle. Systemic insecticides kill soft scales more effectively than armored scales, although suppression of armored scales may be achieved with certain products.
Shore Flies
Identification, Biology and Life Cycle
Shore flies (Scatella stagnalis) and fungus gnats both occur in the damp greenhouse environment. Shore flies are often misidentified as fungus gnats or hunter flies, but they are distinctly different morphologically. Shore fly adults are 1/8 inch (3.1 mm) long and black. They have robust bodies, short antennae and legs, and darkened wings with approximately five distinct white spots, which fungus gnat adults and hunter fly adults do not have. Shore flies, both adults and larvae, feed primarily on algae or decaying organic matter present on the growing medium surface. Like fungus gnats, shore flies breed in moist environments. Adults may be seen resting on plant leaves. Shore fly larvae have been implicated in spreading soil-borne pathogens.
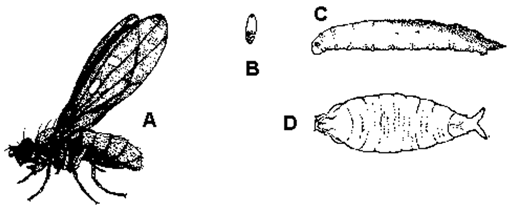
Figure B-10: Shore fly. A-adult, B-egg, C-larva, D-pupa
Shore fly females lay eggs singly on algae. Larvae appear legless and have a breathing tube with two spiracles located at the end of the abdomen. The larvae migrate to the edge of algal mats to pupate.
Scouting
Place yellow sticky cards in the greenhouse, especially in propagation areas. Larvae and adults are often found near algae.
Cultural Control and Mass Trapping
To circumvent shore fly problems, eliminate algae, avoid overwatering, and limit fertilizer run-off. Mass trapping of adult shore flies, with rolls of yellow sticky tape ("hopper tape"), may reduce their numbers.
Biological Control
Rove beetles (Dalotia coriaria) are generalist predators that feed on shore flies, and fungus gnats in the growing medium. Adults are slender, dark brown to black, hairy beetles, about 1/8-inch long, with very short wing covers. The adults can also fly, which helps them disperse throughout the greenhouse. Larvae are cream colored to brown depending upon their age. Both stages are primarily found in the growing medium, hiding in cracks and crevices. The soil dwelling adults and larvae are predaceous. Once established in a greenhouse, they remain year round, but population levels vary depending upon prey populations. Many biological control suppliers sell rove beetles as adults and larvae (all stages). Consult your biological control supplier regarding release rates. Adults are nocturnal, so they are best released in the evening.
Although the entomopathogenic nematode, Steinernema carpocapsae (Millenium) is commercially available for use against shore flies; regulation may be inadequate because of the semi-aquatic habitat in which shore flies live.
Pest Control Materials
For well-established populations, both an adulticide and larvicide may need to be applied. Many insecticides labeled for use against shore flies are insect growth regulators that affect the larval stages.
Thrips
Identification, Biology and Life Cycle
More than a dozen thrips species feed on greenhouse-grown crops. The most important species include the western flower thrips (Frankliniella occidentalis), greenhouse thrips (Heliothrips haemorrhoidalis) and onion thrips (Thrips tabaci). Chilli thrips (Scirtothrips dorsalis) occurs in Florida and Texas and may be introduced on plant material grown there. Unlike western flower thrips, chilli thrips feeds mainly on plant foliage. In 2017, Chilli thrips were detected on landscape hydrangeas with damaged foliage in Barnstable County, MA. Greenhouse thrips adults are commonly found feeding on plant leaves, while western flower thrips adults are concentrated in opened flowers.
Adult thrips are 1/16 inch (2.0 mm) long and slender, with fringed wings. Thrips vary in color from yellow to brown to black. Send specimens to your Extension entomologist to confirm identification.
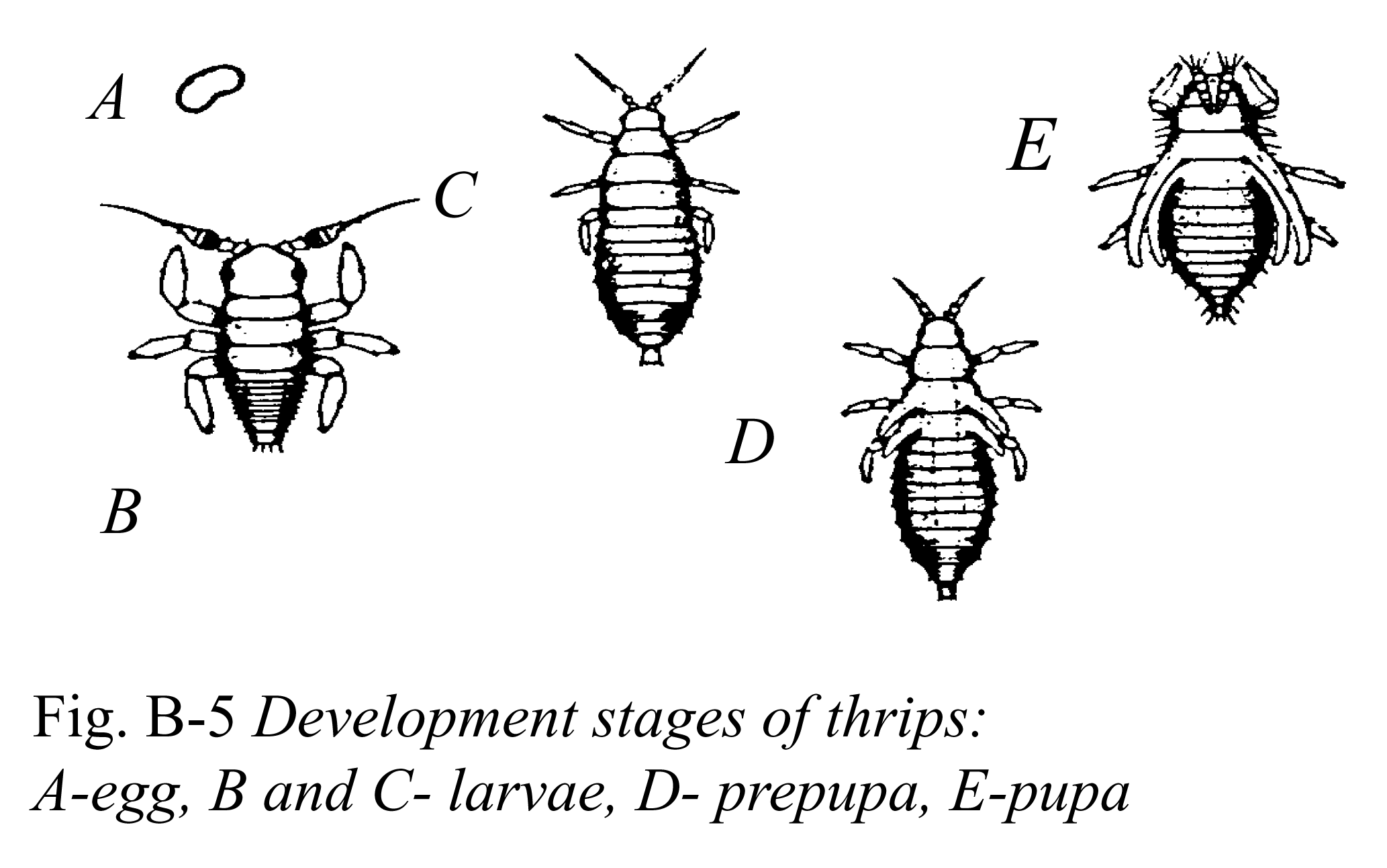
Figure B-11: Development stages of thrips. A-egg, B & C-larvae, D-prepupa, E-pupa
Western Flower Thrips
Adult females insert their saw-like ovipositors into plant leaves and deposit eggs. Eggs hatch in about one week, depending on temperature and plant quality. Larvae or nymphs resemble adults but are wingless. Nymphs feed on plant leaves, newly developed terminal growth and flower buds. First-instar nymphs persist for 1 to 2 days while second-instar nymphs are present for 2 to 4 days. Second-instar nymphs eventually cease feeding and migrate to the base of plants, enter the growing medium, and transition into prepupae, which transform to pupae after 1 to 2 days. Because both prepupae and pupae are typically in the growing medium and not feeding, they are not susceptible to insecticides applied as drenches. Thrips may also pupate in open flowers. The life cycle from egg to adult can be completed in 2 to 4 weeks, with higher temperatures shortening the life cycle. Since thrips are so small, have a high reproductive capacity, and typically feed in unopened terminal or flower buds, they can cause considerable damage to a crop before being detected. Thrips use their piercing-sucking mouthparts to rupture plant tissues, and then feed on the plant fluids that exude from the wounds. The feeding damage appears as silvery or translucent streaks or spots on leaves and flower petals. Thrips also deposit black fecal material on leaves (primarily the underside) and flowers where they feed. Nymphs and adults may be found on leaves, under bud scales, in opened flowers, among pollen sacs, or in leaf axils, depending on the thrips species and host plant. Thrips do not fly well, so they are easily moved throughout a greenhouse on air currents from horizontal air flow (HAF) fans. Thrips may also enter greenhouses through openings such as ridge vents, doors, side walls, and louver vents. They can also be introduced into greenhouses on infested plant material.
Scouting
Several methods may be used to scout or monitor thrips populations. Thrips may be detected by tapping or shaking plants over a white sheet of paper. The dislodged adults and wingless nymphs are noticeable against the white background. For flower-feeding species such as western flower thrips, directly observing flowers or tapping flowers over a white sheet of paper to assess their presence may be an effective monitoring practice. Gently blow into opened flowers to increase thrips activity, thus making it easier to observe them. Select flower colors that are most attractive to thrips (white, yellow, or blue) for this monitoring procedure.
Using yellow or blue sticky cards to capture adult thrips may be more effective and time efficient than the tapping methods described above. Weekly counts of thrips adults on sticky cards help to determine population trends and effectiveness of pest management tactics. Action or damage thresholds are difficult to correlate with thrips counts on sticky cards, particularly when dealing with crops such as impatiens or begonia, which are highly susceptible to impatiens necrotic spot virus. Place sticky cards just above the crop canopy and replace weekly. Move the sticky cards vertically as plants increase in height. As a general rule, use one sticky card per 500 to 1,000 ft2 of greenhouse space. Place sticky cards among susceptible crops and record the number of adult thrips captured on the sticky cards each week. Incorporate this information into a computer database, and use this information to decide whether an insecticide application is warranted over time.
Cultural Control
Many weeds serve as both a refuge for thrips and source of the viruses that are vectored (transmitted) by western flower thrips. These viruses include impatiens necrotic spot virus and tomato spotted wilt virus. Remove weeds, growing medium and plant debris from within and around the greenhouse perimeter to eliminate potential reservoirs of thrips populations. Dispose of any plant debris in garbage containers with tight-sealing lids because thrips may disperse from desiccating plant material onto the main crop. Also, locate all trash receptacles away from greenhouse openings. Screen greenhouse openings such as ridge vents, side walls, louver vents, and walls with cooling pads in order to exclude thrips from greenhouses. Mass trapping with either large yellow sticky cards (16 x 8 inches) or hopper tape placed within 12 feet of the side vents can help mass trap dispersing thrips entering the greenhouse.
Biological Control
Western flower thrips and many other thrips species may be suppressed or regulated on greenhouse-grown crops by releasing predatory mites (e.g. Neoseiulus (Amblyseius) cucumeris, Amblyseius swirskii) or predatory bugs (e.g. Orius species). Minute pirate bugs (Orius species) tend to establish slowly on bedding plants (refer to previous section on using thrips banker plants). Neoseiulus (Amblyseius) cucumeris has been shown to control or regulate thrips populations in ornamentals, herbs and vegetable bedding plants. However, releases must be implemented before thrips populations are high since this predatory mite only feeds on the first instar nymphs. N. cucumeris is often used early in the spring growing season. Another predatory mite, Amblyseius swirskii, toleratates warmer temperatures and may be applied during late spring and summer production. The soil-dwelling predatory mite (Stratiolaelaps scimitus) may feed on thrips pupae in the growing medium in addition to fungus gnat larvae. A single preventive release at planting is usually recommended to supplement aboveground releases of Neoseiulus (Amblyseius) cucumeris or Amblyseius swirskii. The rove beetle, (Dalotia coriaria), is a generalist predator that may feed on thrips pupae, along with fungus gnat and shore fly larvae.
Below are descriptions of the commercially available natural enemies of thrips:
Predatory Mites:
A. Stratiolaelaps scimitus: This soil-dwelling predatory mite is primarily used against fungus gnat larvae. However, it is a generalist predatory mite and may also feed on thrips pupae located in the growing medium, but will not control or regulate populations of thrips by itself, and thus needs to be used in combination with other natural enemies.
B. Neoseiulus (Amblyseius) cucumeris: This predatory mite feeds on thrips residing on leaves and in flowers. It only attacks first instar nymphs since older instar nymphs defend themselves and are more difficult to capture. As a result, it is important to initiate releases preventively before thrips establish high populations. Neoseiulus (Amblyseius) cucumeris feeds on pollen or mites in the absence of thrips. Adults live for approximately three weeks. It is sold in controlled release sachets (paper envelopes) on sticks that contain bran and flour mites (Acarus siro) as a food source. Place sachets within the plant canopy in shaded areas, whenever possible. Direct sunlight, reduces the relative humidity within the sachet, reducing the length of time the mites within the mini-sachet continue reproducing, as the bran dries out.
The predatory mites emerge from the mini-sachets over a four to six-week period. Neoseiulus (Amblyseius) cucumeris is most effective at temperatures >70°F or 21°C (range: 50 to 85°F, or 10 to 29°C) and a relative humidity of 60 to 85%. Avoid direct sunlight and place sachets in the shade. High temperatures and low relative humidity both reduce the egg laying of this beneficial predatory mite and speed up their development so the sachets will not last as long and will reduce their effectiveness.
This predatory mite is also sold in bulk with a bran carrier for broadcast release or for use in breeder piles. Breeder piles are small piles of predatory mites, bran and feeder mites placed on the media surface as a way to distribute the predatory mites.
Some growers start with broadcast releases of predatory mites and wait until the plant canopy in the hanging baskets is sufficient to both shade the mini-sachets and keep the sachets away from contact with the growing media, so the sachets do not become moldy.
Neoseiulus (Amblyseius) cucumeris is not compatible with horticultural oil, but populations of the predatory mite may re-establish on plants after spray residues have dried. Spinosad (Conserve®), abamectin (Avid® and many generic abamectin products), and mixtures of these two pest control materials may reduce N. cucumeris populations. Mixtures of the fungicide thiophanate-methyl (Cleary's 3336™ and other generic products) with the pest control materials spinosad (Conserve®) and abamectin (Avid® and many generic abamectin products) have been shown to be detrimental to N. cucumeris.
C. Amblyseius swirskii: This above-ground predatory mite feeds on both thrips larvae and whiteflies, and has been shown to effectively control or regulate thrips populations on several ornamental crops. Amblyseius swirskii may also feed on eriophyid mite, broad mite, TSM, and pollen in the absence of prey. Amblyseius swirkskii is most effective at warmer temperatures (70°F or 21°C) and 70% relative humidity. It is available in breeding sachets, or can be bulk-applied to the foliage. Consult with your supplier on release rates.
D. Amblyseius limonicus: This predatory mite feds on both thrips larvae and whiteflies. In the absence of prey, it feeds upon pollen. It is not susceptible to diapause and is active at lower temperatures (55° F or 12° C).
Predatory Bugs
Orius species: These predatory bugs, commonly called 'minute pirate bugs', eat immature and adult thrips on leaves and in flowers. As generalists, they also feed on aphids and spider mites. Adults are 1/12 to 1/7 inch (2.2 to 3.8 mm) long with flattened bodies, and are typically black and white. Both adults and nymphs are predaceous. Adults tend to reside in open flowers, where adult thrips are usually located. The light yellow to orange nymphs may be seen on leaves. Orius is most effective at temperatures of 68 to 85°F (20 to 30° C). Release in the early morning or late evening; avoid releases in bright sunlight. Minute pirate bugs are most effective on long-term crops, especially those like ornamental sweet pepper that produce pollen, compared to short-term crops such as bedding plants. Minute pirate bugs may take up to 12 weeks to establish in a greenhouse so they may be applied to thrips banker plants or habitat plants to encourage early development (see previous section on thrips banker plants). They may also undergo diapause (resting period) during short day conditions. Orius is usually sold as adults, which are strong fliers and very active. Tap flowers over a sheet of white paper to detect the adults and nymphs and look for the Orius on the thrips banker plants.
Entomopathogenic Nematodes
Steinernema feltiae: Drench applications of this beneficial nematode against fungus gnat larvae may also be effective against thrips pupae in growing media. Successful programs include a drench application to the growing medium followed by weekly spray or sprench applications (see previous section on fungus gnats for application tips). Apply nematodes in the early morning or late evening to avoid desiccation from ultra-violet light, and when thrips mobility is generally slow. Use blackcloth curtains to minimize ultra-violet (UV) light and heat exposure and turn off artificial lights for at least two hours after applying the nematodes. Spray adjuvants may improve application uniformity, especially on plants with waxy leaves, and allow the nematodes to reach thrips more effectively.
Entomopathogenic Fungi
A. Beauveria bassiana: Beauveria bassiana is a naturally occurring entomopathogenic fungus that is commercially available under the trade names Bioceres®, BotaniGard®, Mycotrol® and Velifer™. The effectiveness of B. bassiana varies depending on relative humidity levels at the plant surface, life stage (egg, nymph, pupa and adult) of the target insect pest, application rate, crop type, spray coverage, light intensity, season and temperature. For example, insects such as thrips and aphids molt their exoskeletons. During warm conditions, these insects molt so rapidly that the spores of B. bassiana are shed along with the exoskeleton so that the entomopathogenic fungus cannot penetrate the insect and initiate an infection. However, the addition of an insect growth regulator such as azadirachtin (Azatin®, Ornazin®, Molt-X™, AzaGuard, Aza-Direct, AzaSol) to the spray solution containing B. bassiana may inhibit molting, thus allowing the fungal spores to penetrate the insect. In addition, applying B. bassiana in early morning may enhance its efficacy. Effective control or regulation of populations of thrips is likely to be achieved when plants are small, when it is easier to obtain thorough spray coverage. Since thrips tend to hide in secluded places, it is essential to thoroughly spray both upper and lower leaf surfaces, and flower and terminal buds. Repeat applications are often needed to maintain low thrips population levels.
B. Isaria fumosoroseus: This naturally occurring insect fungus is commercially available under the trade name Ancora®. This fungus works best at temperatures of 72 to 86°F (22 to 30°C) and requires a relative humidity >75%.
Pest Control Materials
Initiate insecticide applications when populations of thrips are low. Thoroughly cover all plant parts including flowers with the spray solution. Insecticides with either contact or translaminar activity are commonly used to control or regulate populations of thrips. Systemic insecticides generally do not move into flowers where adult thrips typically feed; however, they may suppress or even kill nymphs and adults feeding on leaves. Depending on the thrips population, insecticide applications may be required every 3 to 5 days, especially from spring through late fall. Apply insecticides with equipment that produces small spray droplets that are less than 100 microns in diameter in order to penetrate terminal and flower buds, and open flowers where thrips are usually located. However, in most instances, the use of high-volume application equipment is needed in order to deliver sufficient spray solution to the areas where thrips are located. It is much easier to control or regulate thrips populations when crops are small since this facilitates thorough coverage of all plant parts. Applications to crops with open flowers are generally too late because damage has already occurred.
To delay the onset of insecticide resistance it is imperative to rotate insecticides with different modes of action every 2 to 3 weeks (depending on the season) or after more than one generation. Avoid applying insecticides with repellent properties such as the pyrethroid-based insecticides, since this may cause thrips to disperse throughout the greenhouse.
Weevils
Weevils are the largest group of beetles in the order Coleoptera. They have hardened bodies and distinctly long snouts (mouthparts), and they typically have elbowed antennae. The larvae of many weevil species feed on plant roots while the adults feed on leaves. Many species cannot fly because their wing pads are fused together. The larvae of most species, especially those considered pests of greenhouse-grown crops, are white, grub-like, usually legless and cylindrical. They have well-defined heads and forceful mouthparts.
Black Vine Weevils
Identification, Biology and Life Cycle
A number of weevil species may be encountered in greenhouses, but the black vine weevil (Otiorhynchus sulcatus) is the most important weevil species found in greenhouses and nurseries in the Northeast. Black vine weevil is 3/8 inch (9.0 mm) long, brown-to-black in color with yellow-brown markings on the wing covers, with ridges extending down the length of the abdomen. The surface of the abdomen has distinct rows of minute punctures. Although black vine weevil adults cannot fly, they can crawl through greenhouse openings.
Outdoors, adult female weevils lay eggs in soil or other growing medium near host plants from July through August. Adults can live more than one year, often laying 200 to 400 eggs the first year and over 400 eggs the second year. Eggs hatch in about two weeks. Larvae are white and legless grubs with yellow-brown heads. The larvae have six instars, and when mature are 1/2 inch (13.0 mm) long. Black vine weevil larvae feed on the roots of host plants and then overwinter as full-grown larvae. Pupation occurs in cells that they excavate in the growing medium. Emerging adults are all female. Weevil outbreaks may occur when infested nursery stock is introduced into greenhouses.
Cultural Control
Adults cannot fly but may invade heated structures to overwinter, or may enter outdoor production areas from surrounding vegetation or be accidentally brought in on infested plant material. Avoid black vine weevil infestations by buying nursery stock certified weevil-free. Closely inspect incoming plants to exclude weevils from production areas. Handpick adults if only a few plants are infested in retail settings.
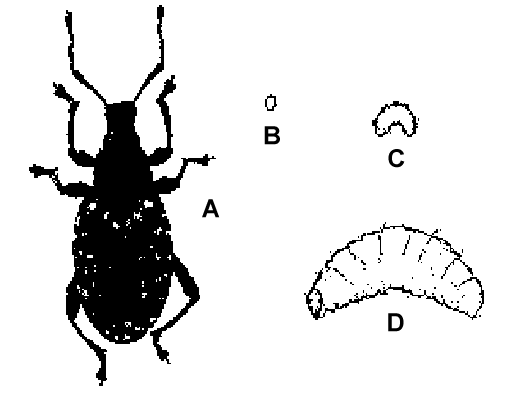
Figure B-12: Black vine weevil. A-adult, B-egg, C & D-larvae
Scouting
Since the larvae are active in the growing media and adults feed at night by notching the edges of leaves, infestions may go unnoticed until significant damage has occurred. Although adults are active at night, they may be monitored by placing horizontal sticky boards on benches to trap adults. Check plant debris and soil or growing medium around plants for the presence of adult black vine weevils. During the day, adults stay at the base of host plants such as astilbe, bergenia, epimedium, helleborus, heuchera, heucherella, hosta, physostegia, phlox, primula, saxifrage, sedum and tricyrtis. Larvae feed on roots, causing leaf yellowing, plant stunting and wilting. If plants exhibit these symptoms, check the root system and surrounding root ball for the presence of larvae. Immediately discard heavily infested plants.
Biological Control
Black vine weevil larvae are susceptible to nearly all commercially available species of entomopathogenic nematodes. Some of the commercially available species include Heterorhabditis bacteriophora, Steinernema kraussei (Nemasys L) and Steinernema carpocapsae which are applied as drenches to the growing medium. Apply the nematodes to moist soil in the early morning or evening to avoid heat and direct sunlight. The nematodes are attracted to, enter and kill all larval stages and pupae, but not eggs or adults.
Heterohabditis bacteriophora is applied to the soil with a minimum temperature of 57°F (14°C). The infected larvae turn brick red to maroon. Steinernema kraussei is effective in a wide range of temperatures from 41 to 86°F (5 to 30°C); infected larvae or pupae turn dark yellow. Consider other Steinernema species if soil temperatures exceed 60°F (15°C); infected larvae or pupae remain creamy yellow-white but are easily identifed as being infected by observing nematode movement through the cutile with a hand lens. These nematodes can be applied from January to May and from August until November to target the larvae. The nematodes are active up to 6 weeks after their application. Repeated applications may be needed.
Pest Control Materials
It may be necessary to apply an insecticide drench to protect adjacent plants. Drench applications of the bifenthrin (Talstar N Granular®) have been shown to effectively kill black vine weevil larvae in containers and in growing medium. Applications must be made when populations are low. Apply insecticides to potential sources of infestation in May when adults begin to emerge from the growing medium. The goal is to kill adult females before they lay eggs.
Whiteflies
Identification, Biology and Life Cycle
The primary whitefly species in greenhouses include the greenhouse whitefly (Trialeurodes vaporariorum) and sweet potato whitefly (Bemisia tabaci), which was formally called the silverleaf whitefly (Bemisia argentifolii). Another whitefly species, bandedwinged whitefly (Trialeurodes abutilonia), may enter greenhouses in the fall. None of the whitefly species mentioned here are able to survive outdoors during New England winters.
Plant material located outside may become infested when winged adults migrate out of greenhouses through openings (e.g., ridge vents, sidewalls and louver vents) as outdoor temperatures increase. Planting infested greenhouse crops outdoors also contributes to infesting outdoor plant material. Whitefly adults, present on weeds or infested host plants outdoors, may migrate into greenhouses in the fall. Nymphs and adults are typically located on the underside of plant leaves. Both nymphs and adults have piercing-sucking mouthparts, which are used to feed on plant fluids. Nymphs may secrete copious amounts of honeydew (clear, sticky liquid) that serves as a growing medium for black sooty mold fungi. Severe infestations of whiteflies may actually defoliate plants. The life cycles of the two primary whitefly species are similar, consisting of eggs, nymphs, pupae (fourth instar nymph) and adults. Development from egg to adult takes 14 to 40 days, depending on temperature, host plant and whitefly species.
Below are descriptions of the whitefly species commonly found in New England greenhouses. The different species are best distinguished by examining the pupal stage found on the leaf undersides.
Greenhouse Whitefly
Greenhouse whitefly adults are most active at temperatures around 75°F (24°C). Adults are winged, white, and 1/16 inch (2.0 mm) long. Greenhouse whitefly adults hold their wings flat, parallel to the top of the body. Females lay more than 20 eggs in a small circle. Newly laid eggs are white and eventually turn gray. Young nymphs (crawlers) are white, have legs and antennae, and move short distances before locating suitable places to initiate feeding. More mature nymphs (third and fourth instars) are typically found on the lower leaves. Pupae do not feed, and have distinct visible red eyes. Greenhouse whitefly pupae may have long waxy filaments encircling the outer edge, and are elevated in profile with vertical sides, resembling "cakes" on leaf surfaces.
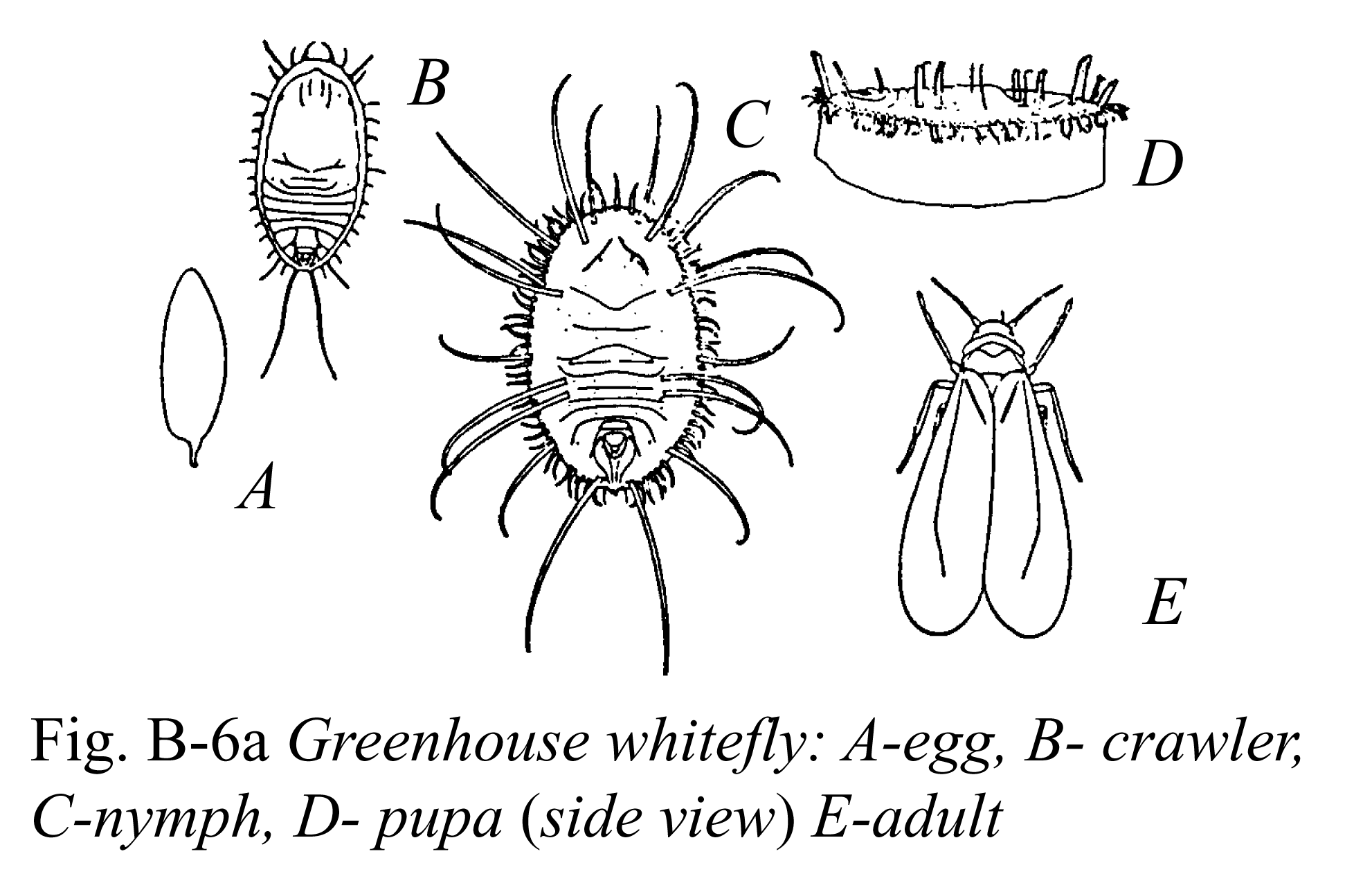
Figure B-12: Greenhouse whitefly. A-egg, B-crawler, C-nymph, D-pupa (side view), E-adult
Sweet Potato Whitefly
Sweet potato whitefly adults are yellow, and smaller than greenhouse whitefly. Their wings are tilted, and held roof-like over their bodies. Adult females live up to six weeks, and produce up to 200 eggs, which are randomly laid in small clusters on new plant growth. Newly laid eggs are white and then turn amber-brown. Young nymphs (crawlers) have legs and antennae, and move short distances before locating suitable places to initiate feeding. More mature nymphs (third and fourth instars) are typically found on the lower leaves. Sweet potato whitefly nymphs are yellow, oval and dome-shaped, and do not have long waxy filaments.
A new biotype of B. tabaci, the Q-biotype, was reported in the U.S. in 2006. This biotype may be problematic because it is known to be resistant to a number of commonly used insecticides including imidacloprid (Marathon®), thiamethoxam (Flagship®), acetamiprid (TriStar™), and the insect growth regulators buprofezin (Talus®) and pyriproxyfen (Distance®). This biotype also vectors leaf curl virus of tomatoes. Submit specimens or samples to specialists for genetic testing because it is difficult to determine which biotype is present based on visual inspection.
Silverleaf whitefly Greenhouse whitefly
Figure B-13: Silverleaf and greenhouse whitefly nymphs
Winged adults emerge in 1 to 2 weeks through T-shaped apertures on the top of pupal cases, which remain attached to leaf surfaces. Empty pupal cases may be present on old or senescing leaves, and may be mistaken for live whitefly nymphs. Adult females initiate egg-laying 2 to 3 days after emerging. Sweet potato whitefly B-biotype adults prefer temperatures >80°F (26°C).
Bandedwinged Whitefly
Bandedwinged whitefly is not an important whitefly species in New England greenhouses. However, adults may be captured on yellow sticky cards in the fall and cause concern among greenhouse growers. In the North, this whitefly species can only survive the winter inside greenhouses. Outdoors, bandedwinged whitefly may be found on weeds such as ragweed (Ambrosia species) and velvetleaf (Abutilon theophrasti).
Adults are yellow in color with a green tinge on the thorax. The front wings are marked with two zigzag, smoky gray bands. When the wings are folded over the body, these lines appear to be continuous from wing to wing. Hind wings lack bands. Waxy filaments encircle the outer edge of fourth instar nymph (pupae) bodies, and a distinct darkened line extends down the center of the body. This trait helps distinguish bandedwinged whitefly from other whitefly species.
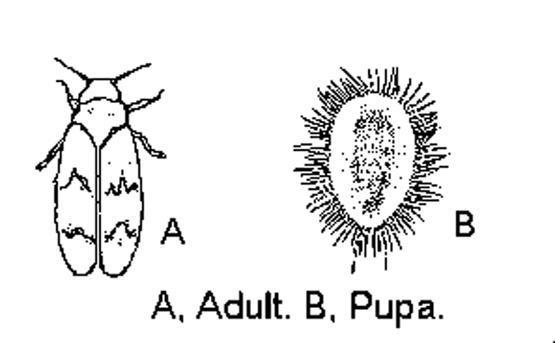
Figure B-14: Bandedwinged whitefly. A-adult, B-pupa
Scouting
Visually inspect incoming plant material for the presence of whiteflies. Once a crop is established, monitor weekly by checking the undersides of 1 to 2 leaves on 10 to 20 plants throughout the greenhouse to detect eggs, nymphs and pupae.
Use yellow sticky cards to capture whitefly adults. Put sticky cards just above the crop canopy and replace weekly. Raise sticky cards as plants grow. As a general rule, use one sticky card per 500 to 1,000 ft2 of greenhouse space. Use more sticky cards for susceptible crops. Count the number of adult whiteflies captured on sticky cards weekly and record this information in a computer database. Use this information to decide if natural enemy releases or insecticide applications are needed.
Cultural Control
Avoid overfertilizing crops as this increases their attractiveness to adult whiteflies. Whiteflies may be introduced into greenhouses on infested cuttings or plants arriving from outside sources. Residual or carryover plants from previous crops and stock plants may also be sources of whiteflies. Using appropriate sanitation practices like weed removal helps alleviate whitefly problems in subsequent cropping cycles.
Biological Control
Two parasitoid species commercially available for controlling or regulating whitefly populations in greenhouses are Encarsia formosa and Eretmocerus eremicus. The predatory mite Amblyseius swirskii mentioned previously under thrips, also feeds upon whitefly eggs and nymphs. The predatory beetle, Delphastus pusillus attacks all stages of whiteflies.
Below are descriptions of these natural enemies.
Whitefly parasitoids
A. Encarsia formosa: This parasitoid, commercially available since the 1970s, is effective against the greenhouse whitefly, particularly in long-term (more than four months) crops. Adult female parasitoids may also feed on young nymphs. Females lay eggs in nymphs; larvae emerge from eggs and consume the internal contents of the whiteflies. Larvae eventually pupate, and emerging adults create a circular hole with their mouthparts, which is used to exit the parasitized whitefly. Parasitized greenhouse whiteflies are turn black as the wasp inside it pupates. Parasitized sweet potato whitefly turn tan to brown. Encarsia formosa is most effective at 70 to 80°F (21 to 26°C), and 50 to 80% relative humidity. Adults don't fly when ambient air temperatures are below 65°F (18°C) and survival is reduced at temperatures >86°F (30°C).
For long-term crops (5 months or longer) infested with greenhouse whitefly, releases of E. formosa are more efficient because it reproduces better on greenhouse whitefly than E. eremicus, which tends to act as a predator by host-feeding on the nymphs. Once E. formosa populations establish in greenhouses, only occasional releases may be needed. Use of E. formosa for control or regulation of greenhouse whitefly populations is recommended for long-term plantings such as conservatories; greenhouse-grown vegetables such as tomatoes, peppers and cucumbers, whose production time exceeds 20 weeks; or crops such as cut flowers since the foliage is not sold.
Remove yellow sticky cards before releasing E. formosa in order to avoid capturing adults on the cards. Replace sticky cards 3 to 4 days following release. Most E. formosa are commercially available as pupae glued to paper cards. Suspend the cards in the lower canopy of plants to avoid desiccation from direct sunlight. Adults emerge from the pupae and fly upward. Introduce cards weekly starting when whiteflies are first detected. In general, for most crops, continue making releases until 80% of the whitefly population has been parasitized. When scouting, look for the distinct black parasitized greenhouse whitefly pupae. Encarsia formosa is very sensitive to direct sprays and even dried residues of products including pyriproxyfen (Distance®), spinosad (Conserve®), chlorfenapyr (Pylon®), acetamiprid (TriStar™) and pyridaben (Sanmite®).
B. Eretmocerus eremicus: This parasitoid has been commercially available since the 1990s for control or regulation of the sweet potato whitefly on poinsettia. It tolerates warm temperatures since it is native to desert areas of California and Arizona. Besides directly parasitizing whitefly nymphs, Eretmocerus eremicus adult females kill nymphs by host feeding, which may actually maintain whitefly populations at low levels. E. eremicus attacks both sweet potato whitefly B and greenhouse whitefly.
E. eremicus is shipped as pupae that are either glued to paper cards or in bottles. E. eremicus is also sold in mixed products with Encarsia. Do not release in direct sunlight. Contact biological control suppliers for information on release rates. Prior to release, remove yellow sticky cards, which attract and capture the emerging parasitoids. Replace yellow sticky cards four days after releases have been made. Development and activity are optimized at 77 to 84°F (25 to 29°C). E. eremicus is inactive at temperatures >86°F (30°C). When scouting, look for parasitized whiteflies. Greenhouse whitefly pupae are yellow while sweet potato whitefly pupae are yellow-brown. In general, E. eremicus is more tolerant of exposure to pest control materials than E. formosa.
In short-term floral crops (less than four months) with lower whitefly thresholds, or crops like poinsettia, E. eremicus is recommended. Releases must begin when plants are potted or when they emerge as seedlings, and should continue during the cropping cycle.
Whitefly Predators
A. Amblyseius swirskii feeds on whitefly eggs and nymphs as well as thrips. It also feeds on pollen. This predatory mite is most effective at warmer temperatures (70°F or 21°C) and a relative humidity of 70%. It is available in breeding sachets, or in bulk to be applied to plant leaves. This predatory mite is compatible with whitefly parasitoids. Consult with your supplier for release rate information.
B. Delphastus pusillus is a small (1.3 to 1.4 mm long) dark brown-to-black predatory beetle that attacks all stages of whiteflies, but prefers eggs and nymphs. Delphastus adults and larvae are predaceous. Optimum temperatures are 75 to 80°F (24 to 27°C), and adults do not fly at temperatures below 55°F (13°C). Release in the early morning or evening. Consult with your supplier on release rates. Delphastus avoids feeding on parastisized whiteflies, so is compatible with the parasitic wasps described above.
Entomopathogenic Fungi
A. Beauveria bassiana: This fungus is commercially available for control or regulation of whitefly populations. Applications must be initiated before whitefly populations are high. Infected whitefly larvae or pupa will become discolored, turning brown or pink as the insect's body cavity is filled with the insect-killing fungus.
B. Isaria fumosoroseus: This fungus is available under the trade name of Ancora®. It attaches to whitefly eggs, nymphs, pupae and adults. I. fumosoroseus requires a relative humidity is 75% or higher and temperatures of 72 to 86°F (22 to 30°C).
Pest Control Materials
The two application methods for pest control materals (insecticides) to control or regulate populations of whiteflies on greenhouse-grown crops are (1) foliar applications directed at adults and nymphs; and (2) systemic insecticides applied to the growing medium to control or regulate nymphal populations. Whitefly eggs and pupae tolerate most insecticides whether used as sprays or drenches, but horticultural oils kill all the life stages if thorough coverage is achieved. Similarly, when using contact insecticides, it is imperative to thoroughly cover leaf undersides. Repeat applications may be needed over a 2- to 3-week period, depending on the extent of the whitefly population and residual activity of the insecticide. Translaminar insecticides may control or regulate whitefly populations for extended periods of time. Start applying systemic insecticides when plants are actively growing to increase uptake of the active ingredient. After systemic insecticide application, it may take several days to weeks before whitefly populations start to decline. This may vary, depending on the water solubility of the systemic insecticide, plant age and growing medium.
Additional Pests
Millipedes
Millipedes are not insects, but belong to the class Diplopoda. They may feed on decaying plant material and may sometimes feed upon young seedlings' roots, especially in ground or soil bed production. Most species curl into a coil when disturbed. They have two pairs of legs per body segment, and short antennae.
Springtails
Springtails are very small (1/5 inch or 5 mm long) wingless insects, and are brown to purple. They have a distinct spring-like apparatus (furcula) at the tip of their abdomen that allows them to jump. They are commonly seen on or in moist growing medium in containers, especially if plants are overwatered. Springtails may be found on potato disks used to monitor for fungus gnat larvae. They are sometimes misidentified as thrips. Springtail females lay eggs in growing medium or soil underneath benches. As they mature, "nymphs" change color and grow. Springtails are primarily scavengers, feeding on decaying organic matter, algae or fungi. However, some species prey on other springtails or nematodes. In general, springtails are not considered a greenhouse pest because they do not feed on plants.
Slugs and Snails
Identification, Biology and Life Cycle
Slugs and snails are not arthropods but are classified as mollusks, closely related to oysters and clams. Snails have hard-shell coverings that protect their bodies. Slugs do not have an outer hardened covering, but they are covered with a copious amount of mucous-like slime that protects their bodies from desiccation. Slugs and snails vary in length from 3/4 to 1-1/2 inches (2 to 4 cm). Their color ranges from pale yellow to lavender to purple. Slugs and snails lay translucent pearl-shaped eggs in clusters of 20 to 100 in cool, moist locations such as in soil or growing medium, underneath mulch, boards, and/or plant pots. Eggs hatch in less than 10 days at 50°F (10°C). Young slugs and snails resemble adults but are lighter in color and smaller. They mature in 3 to 12 months, and adults may live a year or more. Slugs contain both male and female organs, and may alternate sexes during adulthood. Self-fertilization is also possible.
Scouting
Inspect damp, moist areas for slugs and their egg clusters, which are covered with a gelatinous shell that gives them a somewhat milky appearance. Slugs and snails feed on a wide range of greenhouse-grown crops at night. They use their chewing mouthparts to create holes in leaves and stems. Feeding damage from slugs and snails may be misdiagnosed as that of caterpillars. However, caterpillars typically leave fecal deposits on plant leaves and stems. Also, slugs and snails eat leaves and stems completely, while caterpillars may leave portions of stems, leaf veins or the epidermal layer untouched. Slugs and snails also leave shiny mucous-like slime trails in areas they have visited. These trails may be more evident when slugs are most active in the evening or early morning hours, or after plants are irrigated.
Cultural Control
Slugs and snails are commonly introduced into greenhouses via infested plant material, unsterilized growing medium (soil and sand) or supplies (flats and (containers) that have been stored outdoors for an extended time period, especially if perched on soil. Sanitation practices such as weed removal in and around the greenhouse and removing debris (boards and old flats) helps reduce problems with slugs and snails. Sterilizing growing medium and thoroughly cleaning containers or flats prior to use may also alleviate slug and snail problems. Slugs and snails tend to avoid crossing copper barriers, so encircling bench posts with strips of copper sheets may prevent both pests from reaching plants on benches.
Pest Control Materials
Pest control materials (molluscicides) or baits are used to control or regulate populations of slugs and snails. They may contain three active ingredients: metaldehyde (Deadline®), methiocarb (Mesurol®), and iron phosphate (Sluggo®). Metaldehyde does not directly kill slugs and snails, but causes paralysis, resulting in secretion of copious amounts of mucous. Slugs and snails become immobile and desiccate. Metaldehyde is most effective on warm, sunny days. In cool, moist conditions, slugs and snails may actually recover from exposure to metaldehyde. Metaldehyde is very sensitive to environmental conditions and decomposes rapidly when exposed to direct sunlight, but newer formulations appear to resist breakdown when exposed to sunlight. The nerve toxin methiocarb interferes with nerve-impulse transmission. It also acts as a stomach poison, which means slugs and snails must consume the material in order to be affected. This material is effective in cool, moist conditions. Both metaldehyde and methiocarb are toxic to dogs and cats. Iron phosphate, a stomach poison, contains an attractant and the heavy metal, iron. Iron is toxic to slugs and snails, reducing their mobility and causing eventual death. Iron phosphate can be used around pets and wildlife.
Sowbugs
Sowbugs are not insects; they belong to the order Isopoda. Sowbugs are commonly found under containers or flats in moist areas such as propagation greenhouses, because they cannot control the loss of moisture from their bodies. They eat mostly decaying organic matter, although they do feed on young plant roots. Sowbugs have a distinctive "armored" appearance, and often roll up into a ball when disturbed. To reduce sowbug numbers, remove weeds, growing medium and plant debris, and other debris like boards from inside and around the greenhouse.
Symphylans
Symphylans are not insects since they belong to the class Symphyla. They are 1/25 to 1/3 inch (1 to 8 mm) long, slender, wingless, white arthropods with 15 to 20 body segments and long antennae. Adults are 3/8 inch (9 mm) long. They resemble centipedes, but they are not predaceous like centipedes. Instead they feed on plant roots and seeds in the growing medium. Extensive or high populations can stunt growth and even kill plants. To determine the presence of symphylans, remove a plant (along with its roots) from the container, place the root ball into a receptacle of water and swirl the contents for several minutes. Any symphylans present will rise and float on the water surface. There is minimal information about control or regulation of symphylan populations. However, avoid overwatering plants in order to create an environment that is not conducive to development of symphylan populations.
Figure B-15: Symphylan
Supplemental References
Albajes, R., M. L. Gullino, J. C. van Lenteren and Y. Elad [eds.]. 1999. Integrated pest and disease management in greenhouse crops. Kluwer Academic Publishers, The Netherlands.
Cloyd, R. A. 2016. Greenhouse pest management. CRC Press. 198 pp
Cranshaw, W and D. Shetlar. 2018. Garden Insects of North America: the Ultimate Guide to Backyard Bugs. Princeton University Press. 2nd edition. 704 pp.
Dreistadt, S. H. 2001. Integrated pest management for floriculture and nurseries. University of California, Statewide Integrated Pest Management Project, Division of Agriculture and Natural Resources. Publication 3402.
Gill, S. and J. Sanderson. 1998. Ball identification guide to greenhouse pests and beneficials. Ball Publishing, Batavia, IL.
Gill, S., R. A. Cloyd, J. R. Baker, D. L. Clement, and E. Dutky. 2006. Pests & diseases of herbaceous perennials: The biological approach. Ball Publishing, Batavia, IL.
Grower Guide: Quality Assurance of Biocontrol Products. Compiled by Rose Buitenhuis, Vineland Research and Innovation Centre, 2014. Available at: https://www.vinelandresearch.com/wp-content/uploads/2020/02/Grower-Guide.pdf
Heinz, K. M., R. G. Van Driesche, and M. P. Parrella [eds.]. 2004. Biocontrol in protected culture. Ball Publishing, Batavia, IL.
Helyer, N., K. Brown and N. D. Cattlin. 2003. A color handbook of biological control in plant protection. Timber Press, Portland, OR.
Van der Ent, S., M.Knapp, J. Kkapwijk, E. Moerman, J. van Schelt, and S. deWeert. 2017. Knowing and recognizing the biology of glasshouse pests and their natural enemies. K Girard and K. Strooback (Eds). Koppert Biological Systems, The Netherlands. 443 pp.
Greenhouse Scout™ Cornell University (ITunes)
Summarizes information on biocontrol of common greenhouse insect pests and an interactive interface for collecting, organizing, and presentation of scouting data, and product application for insect management.
Thomas, C. 2005. Greenhouse IPM with an emphasis on biocontrols. Publication number AGRS-96. Pennsylvania Integrated Pest Management Program, Pennsylvania Department of Agriculture, and Pennsylvania State University, University Park, PA. Order online at: https://extension.psu.edu/greenhouse-ipm-with-an-emphasis-on-biocontrols
Table B4: Pesticide registration and toxicity
This table provides EPA registration numbers for the pesticides most commonly marketed in New England. Not all manufacturers' products are listed. Label information may change. When applying a pesticide, check the product label for the EPA Registration Number for your records and for crop registration, reentry interval and recommended rates.
z Insecticide Resistance Action Committee (IRAC) number and letter indicate that products with the same number and letter (also known as resistance group) have a common mode of action. This classification is based on the guidelines at https://irac-online.org/. For multiple applications to one crop, select products from different resistant groups. For more IRAC group information, see Table B-5 (Mode of Action (MoA) Classification). Products without resistance group code have not been classified by the IRAC.
y Toxicity: C= Caution W=Warning D=Danger
w This product is labeled for application on edible crops or organic-certified production . See labels or supplemental labels for specific list of crops and applications.
v REI = Re-entry Interval, in hours. See labels for details.
Note:
Generic Pesticides: The list of products in this table is not a complete list and may not contain all generic pesticides or others available for greenhouse use. Generic pesticides are generally off-patent versions of a brand name pesticide. Growers will be seeing many more products on the market as pesticide patents expire; this can make choosing a pesticide very confusing. In some cases, many products exist with the same active ingredients such as insecticides and miticides containing imidacloprid, abamectin, bifenthrin, permethrin and acephate. Read labels carefully since there are similarly named products that may lack greenhouse uses, or may have slightly different concentrations of active ingredient compared to the brand-named product.
Table B–5: Mode of Action (MoA) classification
| Mode of Action* | IRAC ** |
Pest Control Materials Trade name (common name) |
Type *** |
Pest Activity (based on label)**** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APH | CAT | FG | LM | MB | SF | SM | TH | WF | ||||
| Acetylcholine esterase inhibitors | 1A | Mesurol (methiocarb) | C | x | x | x | ||||||
| Acetylcholine esterase inhibitors | 1B | Orthene (acephate) | C, S, T | x | x | x | x | |||||
| Prolong opening of sodium channels | 3A | Talstar (bifenthrin) | C | x | x | x | x | x | x | x | x | |
| Prolong opening of sodium channels | 3A | Decathlon (cyfluthrin) | C | x | x | x | x | x | x | |||
| Prolong opening of sodium channels | 3A | Tame (fenpropathrin) | C | x | x | x | x | x | ||||
| Prolong opening of sodium channels | 3A | Mavrik (tau-fluvalinate) | C | x | x | x | x | x | ||||
| Prolong opening of sodium channels | 3A | Scimitar (lambda-cyhalothrin) | C | x | x | x | x | x | x | x | ||
| Prolong opening of sodium channels | 3A | Astro (permethrin) | C | x | x | x | x | x | x | |||
| Prolong opening of sodium channels | 3A | PyGanic (pyrethrins) | C | x | x | x | x | x | x | x | x | x |
| Nicotinic acetylcholine receptor modulators | 4A | TriStar (acetamiprid) | C, S, T | x | x | x | x | x | x | x | ||
| Nicotinic acetylcholine receptor modulators | 4A | Safari (dinotefuran) | C, S, T | x | x | x | x | x | x | |||
| Nicotinic acetylcholine receptor modulators | 4A | Marathon (imidacloprid) | C, S, T | x | x | x | x | x | x | |||
| Nicotinic acetylcholine receptor modulators | 4A | Flagship (thiamethoxam) | C, S, T | x | x | x | x | x | x | |||
| Nicotinic acetylcholine receptor modulators | 4D | Altus (flupyradifurone) | C, S, T | x | x | x | x | x | ||||
| Nicotinic acetylcholine receptor disruptor agonists and GABA chloride channel activator | 5 | Conserve (spinosad) | C, I, T | x | x | x | x | |||||
| GABA chloride channel activators | 6 | Avid (abamectin) | C, T | x | x | x | x | x | ||||
| Juvenile hormone mimics | 7A | Enstar AQ (kinoprene) | C | x | x | x | x | x | ||||
| Juvenile hormone mimics | 7C | Distance/Fulcrum (pyriproxyfen) | C, T | x | x | x | x | x | x | |||
| Selective-feeding blockers/chordotonal organ TRPV channel modulators | 9B | Endeavor (pymetrozine) | C, I, T | x | x | |||||||
| Selective-feeding blockers/chordotonal organ TRPV channel modulators | 9B | Rycar (pyrifluquinazon) | C, I, T | x | x | x | ||||||
| Selective-feeding blockers/chordotonal organ TRPV channel modulators | 9D | Ventigra (afidopropen) | C, I, T | x | ||||||||
| wth and embryogenesis inhibitors | 10A | Novato (clofentezine) | C | x | ||||||||
| wth and embryogenesis inhibitors | 10A | Hexygon (hexythiazox) | C | x | ||||||||
| wth and embryogenesis inhibitors | 10B | TetraSan (etoxazole) | C, T | x | ||||||||
| Disruptors of insect midgut membranes | 11A | Gnatrol (Bacillus thuringiensis subsp. israelensis) | I | x | ||||||||
| Disruptors of insect midgut membranes | 11A | Dipel (Bacillus thuringiensis subsp. kurstaki) | I | x | ||||||||
| Oxidative phosphorylation uncouplers | 13 | Pylon (chlorfenapyr) | C, T | x | x | x | x | |||||
| Chitin synthesis inhibitors | 15 | Adept (diflubenzuron) | C | x | x | x | x | x | ||||
| Chitin synthesis inhibitors | 15 | Pedestal (novaluron) | C, T | x | x | x | x | |||||
| Chitin synthesis inhibitors | 16 | Talus (buprofezin) | C | x | x | |||||||
| Chitin synthesis inhibitors | 17 | Citation (cyromazine) | C | x | x | x | ||||||
| Ecdysone agonists | 18 | Intrepid 2F (meth-oxyfenozide) | I | x | ||||||||
| Mitochondrial electron transport inhibitors | 20B | Shuttle O (acequinocyl) | C | x | ||||||||
| Mitochondrial electron transport inhibitors | 20D | Floramite SC (bifenazate) | C | x | ||||||||
| Mitochondrial electron transport inhibitors | 21A | Magus (fenazaquin) | C | x | x | |||||||
| Mitochondrial electron transport inhibitors | 21A | Akari (fenpyroximate) | C | x | x | |||||||
| Mitochondrial electron transport inhibitors | 21A | Sanmite SC (pyridaben) | C | x | x | |||||||
| Mitochondrial electron transport inhibitors | 21A | Hachi-Hachi SC (tolfenpyrad) | C | x | x | x | x | x | ||||
| Mitochondrial electron transport inhibitors | 25 | Sultan (cyflumetofen) | C | x | ||||||||
| Lipid biosynthesis inhibitors | 23 | Savate (spiromesifen) | C, T | x | x | |||||||
| Lipid biosynthesis inhibitors | 23 | Kontos (spirotetramat) | C, S, T | x | x | x | x | x | ||||
| Selective activation of ryanodine receptors | 28 | Mainspring GNL (cyantranilprole) | C, S, T | x | x | x | x | x | ||||
| Selective activation of ryanodine receptors | 28 | Acelepryn (chlorantraniliprole) | C, S, T | x | ||||||||
| Selective activation of ryanodine receptors | 28 | Sarisa (cyclaniliprole) | C, S,T | x | x | x | x | x | x | |||
| Selective feeding blocker/Chordotonal organ modulator | 29 | Aria (flonicamid) | C, S, T | x | x | x | x | |||||
| Dessiccators or Membrane disruptors | M-Pede (potassium salts of fatty acids) | C | x | x | x | x | x | |||||
| Dessiccators or Membrane disruptors | Triact (clarified hydrophobic extract of neem oil) | C | x | x | x | x | ||||||
| Dessiccators or Membrane disruptors | Ultra-Pure Oil/SuffOil-X (mineral oil) | C | x | x | x | x | x | x | x | |||
| Unclassified by IRAC | Ancora (Isaria fumosoroseus) | C | x | x | x | x | x | x | ||||
| Unclassified by IRAC | Azatin/Molt X (azadirachtin) | C | x | x | x | x | x | x | x | x | ||
| Unclassified by IRAC | BotaniGard ES/Mycotrol ESO (Beauveria bassiana) | C | x | x | x | x | x | |||||
| Unclassified by IRAC | Grandevo (Chromobacterium subtsugae) | C | x | x | x | x | x | |||||
| Unclassified by IRAC | Met 52 (Metarhizium brunneum) | C | x | x | x | |||||||
| Unclassified by IRAC | Overture (pyridalyl) | C, I, T | x | x | ||||||||
| Unclassified by IRAC | Venerate (Burkholderia spp. Strain A396) | C, I | x | x | x | x | x | |||||
| Multiple Modes of Action | 5 + 4C | XXpire (spinetoram + sulfoxaflor) | C,S,T | x | x | x | x | x | x | |||
| Multiple Modes of Action | 28 + 29 | Pradia (cyclaniliprole + flonicamid) | C,S,T | x | x | x | x | x | x | |||
Key for table B-5:
*Modes of action. See IRAC's Web site for more information: www.irac-online.org
Group 1 (Acetylcholine Esterase Inhibitors): Inhibit the enzyme cholinesterase (ChE) from clearing the acetylcholine (ACh) transmitter. This prevents termination of nerve impulse transmission and results in an accumulation of acetylcholine leading to hyperactivity, respiratory failure, exhaustion of metabolic energy, and death.
Group 3 (Sodium Channel Blockers): Destabilize nerve cell membranes by working on the sodium channels in the peripheral and central nervous system; slowing down or preventing closure. This results in stimulating nerve cells to produce repetitive discharges, eventually leading to paralysis and death.
Group 4 (Nicotinic Acetylcholine Receptor Disruptors): Act on the central nervous system, causing irreversible blockage of the post-synaptic nicotinergic acetylcholine receptors leading to disruption of nerve transmission and uncontrolled firing of nerves. This results in rapid pulses from a steady influx of sodium, leading to hyperexcitation, convulsions, paralysis and death.
Group 5 (Nicotinic Acetylcholine Receptor Agonists): Disrupt binding of acetylcholine at nicotinic acetylcholine receptors located at the post-synaptic cell junctures, and negatively affect the gamma-amino butyric acid (GABA) gated ion channels.
Group 6 (GABA Chloride Channel Activators): Affect gamma-amino butyric acid (GABA) dependent chloride ion channels by increasing membrane permeability to chloride ions leading to inhibition of nerve transmission, paralysis and death.
Group 7 (Juvenile Hormone Mimics): Arrest development by causing insects to remain in a young or immature stage primarily by inhibiting metamorphosis, or change in form. As a result, insects are unable to complete their life cycle.
Group 9 (Selective Feeding Blockers): Inhibit feeding behavior of insects by interfering with neural regulation of fluid intake in the mouthparts.
Group 10 (Growth and Embryogenesis Inhibitors): Disrupt the formation of the embryo during development, or inhibit larval maturation. However, the specific mode of action and target site of activity are still not known.
Group 11 (Disruptors of Insect Midgut Membranes): Bind to specific receptor sites on the gut epithelium resulting in degradation of the gut lining and eventual starvation of the insect. Crystals release protein toxins (endotoxins) that bind to the mid-gut membrane receptor sites creating pores or channels. This paralyzes the digestive system and ruptures the midgut cell walls allowing ions to flow through the pores disrupting potassium and pH balances. As a result, the alkaline contents of the gut spill into the blood resulting in gut paralysis and death.
Group 13 (Oxidative Phosphorylation Uncouplers): Inhibit oxidative phosphorylation at the site of dinitrophenol uncoupling, which distrupts the formation or synthesis of adenosine triphosphate (ATP).
Groups 15, 16, and 17 (Chitin synthesis inhibitors): Inhibit formation of chitin, an essential component of an insect's exoskeleton, affecting the firmness and elasticity of the cuticle. Insects die during the process of molting from one life stage to the next.
Group 18 (Ecdysone receptor): Strong evidence shows that action at this hormone is responsible for insecticidal effects.
Groups 20, 21 and 25 (Mitochondria Electron Transport Inhibitors): Inhibit Complex (site) I electron transport or act on the NADH-CoQ reductase site, or bind to the Qo center of Complex III in the mitochondria reducing energy production by preventing the synthesis of adenosine triphosphate (ATP).
Group 23 (Lipid Biosynthesis Inhibitors): Block the production of lipids, which are a group of compounds made up of carbon and hydrogen including fatty acids, oils and waxes. Disrupts cell membrane structures and reduces sources of energy.
Group 28 (Ryanodine Receptor Modulators): Disrupt nerve and muscle action.
Group 29 (Chordotonal Organ Modulators): Disrupt nerve action.
Desiccation or Membrane Disruptors: Damage the waxy layer of the exoskeleton of soft-bodied insects and mites by altering the chitin so that it cannot hold fluids resulting in desiccation, or smother insects by covering their breathing pores (spiracles).
** IRAC (Insecticide Resistance Action Committee) designation, which appears on many product labels.
|
***Type codes: C = contact I = ingested S = systemic T = translaminar |
****Pest Activity Codes: APH = aphids CAT = caterpillars FG = fungus gnats LM = leafminers MB = mealybugs SF = shoreflies SM = spider mites TH= Thrips WF= Whiteflies |
Using Insecticides and Miticides in the Greenhouse
Development of Pesticide Resistance
Pesticide resistance is the reduction in the pest susceptibility to an active ingredient. Resistance is genetic in nature. An individual insect or mite pest does not become resistant or acquire resistance to pesticides (insecticides and miticides) during its lifetime —that is, within one generation. Instead, resistance occurs as a result of frequent applications of a pesticide with the same mode of action— a process known as selection pressure. This leads to some individuals in a pest population surviving and transferring traits for resistance to the next generation, which may enrich the gene pool with resistant genes. Repeated applications with one type of pesticide or pesticides that target have the same mode of action eventually remove almost all the susceptible individuals from a pest population, leaving only individuals with genes for resistance.
Each time an insect or mite pest population is exposed to a pesticide, there is potential selection for resistance, which increases the frequency or proportion of resistant genes within that population. Any traits that provide an adaptive advantage include rare versions of genes that diminish sensitivity to a particular pesticide, or altered gene expression that results in amplification of commonly existing genes.
Insect pests can become resistant to insecticides that they have never been exposed to. This happens when two insecticides have similar modes of action. If two (or more) insecticides attack the same target site, the resistance mechanism to one insecticide may provide resistance to the other, although the insect pest may never have been exposed to the second insecticide.
How to Mitigate Pesticide Resistance:
-
Use biological, cultural, and sanitation management strategies.
-
Reduce the use of pesticides whenever possible, particularly those with site-specific modes of action. Scout crops regularly and establish thresholds that justify applications of pesticides.
-
Treat small or localized areas within the greenhouse whenever possible rather than treating the entire greenhouse or entire sections of the greenhouse.
-
Avoid applying persistent pesticides and slow- release-encapsulated formulations. Apply concentrations that are high enough to kill nearly all individuals in a population, and then quickly dissipate.
-
Time pesticide applications to target the most susceptible life stages (i.e. larvae and adults).
-
Avoid pesticide mixtures (mixing two or more pesticides together into a single solution to control or regulate populations of a single pest) except in cases where research has demonstrated improved efficacy. Examples of pesticide mixtures that have resulted in improved efficacy include Tame (fenpropathrin) and Orthene (acephate); Avid (abamectin) and Azatin (azadirachtin); Azatin (azadirachtin) and Talstar (bifenthrin); and Azatin (azadirachtin) and BotaniGard (Beauveria bassiana). Take precautions when tank mixing because phytotoxicity (plant injury) can occur with a mixture even though no problems were observed when either pesticide was applied separately.
For more detailed information on pesticide mixtures refer to the following publication:
Pesticide Mixtures: Understanding Their Use in Horticultural Production Systems. Kansas State University Agricultural Experiment Station and Cooperative Extension Service; Manhattan, KS. MF-3045, April 2012 (Author: R. A. Cloyd). Available online at: https://bookstore.ksre.ksu.edu/pubs/MF3045.pdf
Rotate pesticides with different modes of action. Unless otherwise directed on the pesticide label, switch to a pesticide with a different mode of action within every 2-to-3 pest generations or about every 2-to-3 weeks. Refer to Table B-5 for information about pesticides and their mode of activity. The actual interval of a pesticide rotation depends on the time of year, as temperatures and season influence the duration of insect and mite pest life cycles. For example, warm temperatures often lead to overlapping generations and various life stages present at the same time. As a result, more frequent applications and rotations of pesticides are required. In winter, insect and mite pest development is slower and pesticides may not need to be rotated as often.
-
Use pesticides with broad modes of action whenever possible. Most conventional pesticides kill insect and mite pests by affecting very specific chemical or nervous system pathways (e.g., interfere with nerve transmission or oxidative phosphorylation). The use of pesticides with broad modes of action such as insecticidal soaps, horticultural oils, selective-feeding blockers, insect growth regulators, and microbial organisms (e.g., bacteria and fungi) will avoid or delay the onset of resistance developing in insect and/or pest populations.
-
Note that it is important to rotate pesticides with broad modes of action, as there are certain insect pests that have developed resistance to insect growth regulators and bacterial-based insecticides including Bacillus thuringiensis subsp. kurstaki (Btk).
For more detailed information on resistance management, refer to the following publication:
Resistance Management: Resistance, Mode of Action, and Pesticide Rotation. Kansas State University Agricultural Experiment Station and Cooperative Extension Service; Manhattan, KS. MF-2905, January 2010 (Authors: R. A. Cloyd and R. S. Cowles). Available online at: https://bookstore.ksre.ksu.edu/pubs/mf2905.pdf
How to Successfully Use Pesticides
1. Test the pH of the water before mixing pesticides, and also test the pH of the spray solution. Many pesticides, especially those in the organophosphate chemical class, are not effective when mixed in water with a pH greater than 7. If necessary, use a commercially available acidifier to adjust the pH of water to be neutral (pH 7) or slightly acidic. The pH range in which most pesticides are stable is between 5.8 and 6.5. Always read the label to determine the appropriate pH for specific pesticides.
2. Accurately measure the required amount of pesticide to be used. Measure solid formulations (e.g., wettable powders and soluble powders) using a scale, and liquid formulations using graduated cylinders. Always read the label for more information.
3. After mixing a pesticide with water, apply the solution immediately or within a few hours. Never let a spray solution stand overnight before using.
4. Always apply a pesticide according to label directions. Most pesticide labels contain information on how much to apply in a certain area. This is important in delivering the correct amount of active ingredient to maximize efficacy.
Preventing Phytotoxicity (Plant Injury) From Pesticide Applications
-
Apply pesticides in the early morning or evening. Applications made in the early morning allow plant foliage to dry before temperatures reach 85 to 90°F. Take special precautions when using pesticides containing either petroleum or paraffinic base oil. Always make applications when conditions allow plant foliage to dry quickly.
-
Add surfactants only when recommended on the pesticide label.
-
Avoid pesticide mixtures. A mixture of different pesticides may increase the chances of harming crops.
-
Never use a sprayer for insecticides that was previously used to apply herbicides.
-
Apply pesticides only after crops have been irrigated. Never apply pesticides to plants that are under water stress.
-
Do not apply pesticides with a fertilizer.
-
Never use herbicides (weed killers) within the greenhouse unless they are specifically labeled for use in the greenhouse.
Get Pesticide Labels SDS (Safety Data Sheets), and Related Information from:
Greenbook/C&P Press, Inc.: www.greenbook.net
CDMS, Inc: www.cdms.net
EPA: Pesticide Product Label System: https://www.epa.gov/pesticide-labels/pesticide-product-label-system-ppls-more-information
BioWorks: www.bioworksinc.com
SePRO Corporation (Sepro): www.sepro.com
OHP, Inc.: www.ohp.com
Kelly Registrations Systems, Inc. (pesticide registrations; see state data search on webpage)
www.kellysolutions.com
Organic Materials Review Institute (OMRI): www.omri.org
IRAC Mode of Action Classification: www.irac-online.org
USDA National Organic Program www.ams.usda.gov/nop
Table B-6: Insecticides, Miticides, & Molluscides Products and Rates for Greenhouse Ornamentals
If any information in this table is inconsist ent with the label, then follow the label. Refer to technical representatives for small volumes.Refer to label for rates on edible crops.